What Is Meant By The Statement Enzymes Are Biological Catalysts
Breaking News Today
Mar 21, 2025 · 6 min read
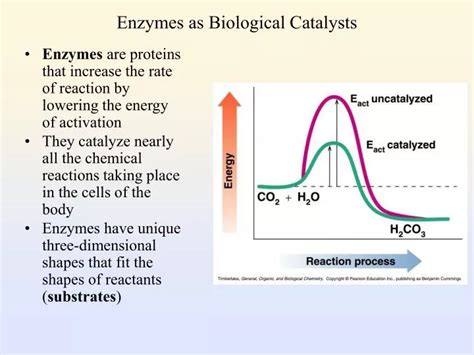
Table of Contents
What is Meant by the Statement: Enzymes are Biological Catalysts?
Enzymes are ubiquitous in biological systems, playing a crucial role in virtually every metabolic process. Understanding their function as biological catalysts is fundamental to comprehending the intricacies of life itself. This in-depth article delves into the meaning of this statement, exploring the properties of enzymes, their catalytic mechanisms, and their significance in various biological contexts.
Understanding Catalysts: Speeding Up Reactions
Before diving into the specifics of enzymes, let's establish a clear understanding of what a catalyst is. A catalyst is a substance that increases the rate of a chemical reaction without itself being consumed in the process. It achieves this by lowering the activation energy of the reaction – the minimum energy required for the reaction to proceed. Think of it like this: a catalyst provides an alternative, lower-energy pathway for the reaction to take place. This results in faster reaction rates, even at lower temperatures. Catalysts are not permanently altered during the reaction; they can participate in multiple reaction cycles.
Key characteristics of catalysts:
- Increased reaction rate: Catalysts significantly accelerate the rate at which reactants are converted into products.
- Unchanged by the reaction: Catalysts are not consumed during the reaction; they emerge unchanged at the end.
- Lowered activation energy: Catalysts achieve their effect by lowering the activation energy barrier.
- Specificity: Some catalysts exhibit specificity, meaning they only catalyze particular reactions or types of reactions.
Enzymes: Biological Catalysts in Action
Enzymes are biological catalysts, meaning they are proteins (or in some cases, RNA molecules called ribozymes) that catalyze biochemical reactions within living organisms. Their catalytic power is extraordinary, often increasing reaction rates by factors of millions or even billions. This remarkable efficiency is essential for maintaining the delicate balance of metabolic processes necessary for life.
What makes enzymes such effective catalysts?
- High Specificity: Enzymes exhibit remarkable specificity, meaning they typically catalyze only one particular reaction or a very small group of closely related reactions. This specificity arises from the precise three-dimensional structure of the enzyme, which forms a unique active site.
- Mild Reaction Conditions: Enzymes operate under relatively mild conditions of temperature and pH, in contrast to many chemical catalysts that require harsh conditions. This is crucial for maintaining the integrity of the cellular environment.
- Regulation: Enzyme activity is highly regulated, allowing organisms to control metabolic pathways in response to changing conditions. This regulation can involve allosteric regulation, covalent modification, or changes in enzyme concentration.
- Efficient Turnover: Enzymes are highly efficient, able to catalyze many reactions per second. This high turnover rate is essential for the rapid processing of substrates in metabolic pathways.
The Enzyme-Substrate Complex: The Heart of Catalysis
The catalytic activity of enzymes relies on the formation of an enzyme-substrate complex. The substrate is the molecule upon which the enzyme acts. The enzyme's active site, a specific region within its three-dimensional structure, binds to the substrate with high affinity. This binding creates the enzyme-substrate complex, bringing the substrate molecules into close proximity and proper orientation for the reaction to occur.
The Induced Fit Model: A Dynamic Interaction
The prevailing model for enzyme-substrate interaction is the induced-fit model. This model posits that the active site is not a rigid, pre-formed structure. Instead, the binding of the substrate induces a conformational change in the enzyme, optimizing the active site for catalysis. This dynamic interaction enhances the enzyme's ability to bind the substrate and facilitate the reaction.
Mechanisms of Enzyme Catalysis
Several mechanisms contribute to the remarkable catalytic efficiency of enzymes:
- Proximity and Orientation: The enzyme brings the substrate molecules into close proximity and in the correct orientation for the reaction to occur, significantly increasing the likelihood of successful collisions.
- Acid-Base Catalysis: Enzyme active sites often contain acidic or basic amino acid residues that participate in proton transfer reactions, facilitating the reaction mechanism.
- Covalent Catalysis: Some enzymes form temporary covalent bonds with the substrate during the reaction, creating a reaction intermediate that is more reactive than the original substrate.
- Metal Ion Catalysis: Many enzymes require metal ions as cofactors for their activity. These ions can participate in redox reactions, stabilize charge distributions, or facilitate substrate binding.
Enzyme Classification and Nomenclature
Enzymes are classified into six main classes based on the type of reaction they catalyze:
- Oxidoreductases: Catalyze oxidation-reduction reactions (e.g., dehydrogenases, oxidases).
- Transferases: Catalyze the transfer of functional groups between molecules (e.g., kinases, transaminases).
- Hydrolases: Catalyze hydrolysis reactions (e.g., lipases, proteases).
- Lyases: Catalyze the addition or removal of groups to or from double bonds (e.g., decarboxylases, hydratases).
- Isomerases: Catalyze isomerization reactions (e.g., mutases, epimerases).
- Ligases: Catalyze the joining of two molecules coupled with the hydrolysis of ATP (e.g., synthetases, carboxylases).
Each enzyme is assigned a unique EC number, a four-digit code that reflects its class, subclass, sub-subclass, and specific activity.
Factors Affecting Enzyme Activity
Several factors influence the rate at which enzymes catalyze reactions:
- Substrate Concentration: Increasing substrate concentration generally increases the reaction rate up to a point, after which the enzyme becomes saturated and the rate plateaus.
- Enzyme Concentration: Increasing enzyme concentration increases the reaction rate proportionally, provided there is sufficient substrate.
- Temperature: Enzymes have an optimal temperature at which they function most efficiently. Higher or lower temperatures can denature the enzyme, reducing its activity.
- pH: Enzymes have an optimal pH range. Extreme pH values can denature the enzyme or alter the charge of amino acid residues in the active site, affecting substrate binding and catalysis.
- Inhibitors: Inhibitors are molecules that reduce enzyme activity by binding to the enzyme and interfering with its function. Inhibitors can be competitive (competing with the substrate for the active site) or non-competitive (binding to a site other than the active site).
- Activators: Activators are molecules that enhance enzyme activity, often by binding to the enzyme and inducing a conformational change that improves substrate binding or catalysis.
The Significance of Enzymes in Biological Processes
Enzymes are indispensable for life, playing a crucial role in a vast array of biological processes:
- Metabolism: Enzymes catalyze the countless reactions involved in metabolic pathways, including glycolysis, the citric acid cycle, and oxidative phosphorylation.
- Digestion: Digestive enzymes break down large food molecules into smaller, absorbable units.
- DNA Replication and Repair: Enzymes are essential for DNA replication, transcription, and repair.
- Protein Synthesis: Enzymes catalyze the formation of peptide bonds during protein synthesis.
- Signal Transduction: Enzymes play key roles in signal transduction pathways, converting extracellular signals into intracellular responses.
- Immune Response: Enzymes are involved in the immune response, contributing to the recognition and destruction of pathogens.
Conclusion: The Irreplaceable Role of Enzymatic Catalysis
The statement "enzymes are biological catalysts" encapsulates the fundamental role of enzymes in life. Their remarkable catalytic efficiency, specificity, and regulation are crucial for maintaining the intricate balance of biochemical reactions within living organisms. From the simplest metabolic pathways to the most complex cellular processes, enzymes are indispensable components of the machinery of life. Understanding their function as biological catalysts provides a cornerstone for comprehending the very essence of biology. Further research into enzyme structure, function, and regulation continues to yield invaluable insights into biological processes and holds immense potential for developing new therapies and technologies.
Latest Posts
Latest Posts
-
Which Sentence Best Describes The Chief Characteristics Of Blank Verse
Mar 21, 2025
-
What Is True Of An Eyeshadow Base Color
Mar 21, 2025
-
En Las Playas De Rincon Puedes Ver
Mar 21, 2025
-
Time Values In Music Are Expressed In
Mar 21, 2025
-
Which Financing Option Has The Highest Overall Costs
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is Meant By The Statement Enzymes Are Biological Catalysts . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
