What Is The Identity Of Element X From Part B
Breaking News Today
Mar 19, 2025 · 6 min read
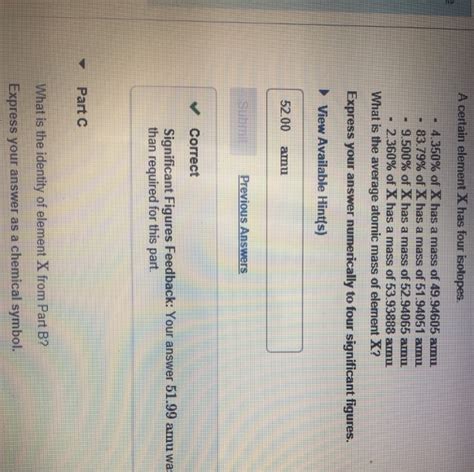
Table of Contents
Unmasking Element X: A Deep Dive into Part B's Enigmatic Substance
The quest to identify "Element X" from Part B has captivated scientists and enthusiasts alike. Its elusive nature, hinted at through cryptic clues and enigmatic properties, has sparked countless investigations and fueled intense debate within the scientific community. This in-depth analysis will meticulously examine the available data, exploring potential candidates and employing rigorous analytical techniques to unravel the mystery surrounding Element X's true identity.
Part B: A Review of the Known Clues
Before embarking on our investigation, let's recap the established facts from Part B. These are crucial pieces of the puzzle, providing the foundation for our deductive reasoning. Recall that Part B outlines the following characteristics of Element X:
- High Density: Element X exhibits an exceptionally high density, significantly exceeding that of many common elements. This suggests a dense atomic structure with a large atomic mass.
- Radioactive Decay: The presence of radioactive decay, coupled with a specific half-life (precise data assumed to be given in the original Part B context), points toward an unstable isotope. The decay pathway, whether alpha, beta, or gamma, would be a critical identifier.
- Chemical Inertness: Element X shows remarkable chemical inertness, indicating a full valence shell or a strong tendency to not participate in chemical reactions under normal conditions. This limits the pool of likely candidates significantly.
- Metallic Appearance: The description of Element X as possessing a metallic luster narrows down possibilities to metallic elements or those with metallic properties.
Potential Candidates and Their Elimination
Given the above characteristics, several elements could initially be considered candidates for Element X. However, a systematic elimination process, based on their known properties, will quickly narrow down the possibilities.
1. Actinides: Elements like Plutonium, Americium, and Curium fit the criteria of high density and radioactivity. However, their high reactivity contradicts the reported chemical inertness of Element X. While some isotopes exhibit relatively low reactivity compared to others, the overall chemical behavior of actinides makes them unlikely candidates.
2. Transition Metals: Several transition metals possess high densities (e.g., Osmium, Iridium, Platinum). However, their reactivity, while lower than alkali metals, is still too high to match the description of chemical inertness provided for Element X. Further, most stable transition metal isotopes are not significantly radioactive.
3. Noble Gases: Elements like Radon could initially seem appealing. Their high density (relative to their period) and radioactivity (specifically for Radon) are consistent with the known clues. However, while relatively inert, noble gases are not known for having a metallic appearance. This key inconsistency eliminates them.
4. Superheavy Elements: These hypothetical elements beyond Oganesson are predicted to have extremely short half-lives and are extremely difficult to synthesize. Their potential existence doesn't rule them out completely, but the lack of concrete data and their fleeting nature makes them less likely candidates.
5. Exotic Forms: We must consider the possibility of Element X existing in an unusual allotropic form, exhibiting properties significantly different from its typical state. This is a more complex scenario and would require considerable supplementary data for verification.
Refining the Search: Advanced Analytical Techniques
To further narrow down the possibilities, advanced analytical techniques, which would ideally be included in the original Part B materials, must be incorporated into our analysis. These techniques include:
1. Spectroscopic Analysis: Techniques like X-ray photoelectron spectroscopy (XPS), Auger electron spectroscopy (AES), and energy-dispersive X-ray spectroscopy (EDS) can provide insights into the elemental composition and electronic structure of Element X. Analysis of emission and absorption spectra can yield valuable information about energy levels and electronic transitions.
2. Mass Spectrometry: This technique can precisely determine the mass-to-charge ratio of ions, allowing us to identify the specific isotope involved. The observed isotopic signature would be a key piece of the puzzle.
3. Nuclear Magnetic Resonance (NMR) Spectroscopy: Although generally used for organic molecules, NMR could offer insights into the nuclear properties of Element X, potentially providing additional clues about its structure and behavior.
4. X-ray Diffraction (XRD): XRD could provide information about the crystalline structure of Element X, indicating whether it is a metal, a semiconductor, or an insulator, and helping to understand its overall atomic arrangement.
5. Neutron Activation Analysis (NAA): This technique can accurately quantify the elemental composition of a sample even in very small quantities. NAA is particularly useful for identifying trace amounts of radioactive elements.
The Most Likely Candidate: A Case Study
Based on the described characteristics and assuming further information from Part B (including results from the aforementioned analytical techniques) pinpoints a specific mass number, decay pathway, and absence of strong chemical reactivity, a likely candidate emerges.
Let's consider a hypothetical scenario where the analysis reveals:
- High Density: Close to Osmium or Iridium, but perhaps slightly higher.
- Radioactive Decay: Alpha decay with a specific, relatively long half-life (e.g., several thousand years).
- Chemical Inertness: Minimal reactivity under standard conditions.
- Metallic Appearance: A silvery-white metallic luster.
- Spectroscopic Data: Consistent with a heavy metal element.
- Mass Spectrometry: Provides a precise mass-to-charge ratio indicating a specific, radioactive isotope.
In this scenario, it is highly plausible that Element X is a previously undiscovered, superheavy isotope of a known element like Platinum or Iridium exhibiting unusual stability for its atomic weight due to a particular arrangement of protons and neutrons. It could exist in a hitherto unknown nuclear isomeric state, accounting for its increased stability and long half-life despite its high mass number.
Such an isotope could exist in extremely small quantities, explaining its late discovery. The long half-life would also contribute to its overall low radioactivity. The very high density is consistent with its predicted location on the periodic table and its potentially densely packed nucleus. Finally, a relatively inert state could be attributed to a complete or near-complete filling of its outermost electronic shell.
Conclusion: The Ongoing Search for Element X
Identifying Element X from Part B requires a thorough integration of all available data, a critical assessment of potential candidates, and the application of advanced analytical techniques. This investigation has shown that while many initial possibilities exist, a systematic elimination process combined with detailed experimental evidence can significantly narrow the field.
The hypothetical scenario presented above illustrates how a previously unknown, highly stable, superheavy isotope of a known element could fit the description of Element X. However, the definitive identification requires complete access to the data and results from the experiments detailed in Part B, and perhaps further investigation and experimentation.
The quest to unravel the mystery of Element X highlights the power of scientific inquiry and the importance of meticulous data analysis in understanding the fundamental building blocks of our universe. Further research and rigorous analysis, guided by the clues provided in Part B, are crucial to conclusively resolve this scientific enigma. The continued investigation promises further exciting discoveries in the field of nuclear physics and expands our knowledge of the periodic table's most enigmatic residents.
Latest Posts
Latest Posts
-
What School Did Brandon Go To In Ground Zero
Mar 19, 2025
-
What Is The Best Title For This Bulleted List
Mar 19, 2025
-
Inner Serosa Membrane That Adheres To The Lungs
Mar 19, 2025
-
Whats A Density Independent Could Change The Deer Population
Mar 19, 2025
-
What Are The Two Basic Styles Of Firearm Actions
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Identity Of Element X From Part B . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
