What Type Of Bond Is Joining The Two Hydrogen Atoms
Breaking News Today
Mar 18, 2025 · 6 min read
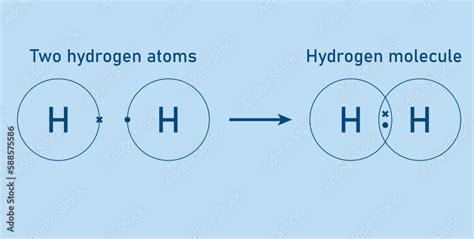
Table of Contents
What Type of Bond is Joining the Two Hydrogen Atoms?
The seemingly simple question, "What type of bond is joining the two hydrogen atoms?" opens a door to a fascinating exploration of fundamental chemistry. While the answer might seem straightforward at first glance – a covalent bond – delving deeper reveals nuances and complexities that highlight the elegance and power of chemical bonding. This article will explore the nature of the hydrogen-hydrogen bond, examining its formation, characteristics, and significance in various contexts.
Understanding Covalent Bonds
Before diving into the specifics of the hydrogen-hydrogen bond, let's establish a firm understanding of covalent bonding itself. A covalent bond is formed when two atoms share one or more pairs of electrons. This sharing allows both atoms to achieve a more stable electron configuration, typically resembling that of a noble gas (a full outer electron shell). This shared electron pair is attracted to the positively charged nuclei of both atoms, creating a strong attractive force that holds them together.
The strength of a covalent bond depends on several factors, including the electronegativity of the atoms involved. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. When two atoms with similar electronegativities bond, the electrons are shared relatively equally, resulting in a nonpolar covalent bond. Conversely, when atoms with significantly different electronegativities bond, the electrons are shared unequally, leading to a polar covalent bond. The resulting molecule possesses a dipole moment, with one end slightly positive and the other slightly negative.
The Hydrogen-Hydrogen Bond: A Nonpolar Covalent Bond
In the case of a dihydrogen molecule (H₂), two hydrogen atoms are joined by a single, nonpolar covalent bond. Each hydrogen atom contributes one electron to the shared pair, completing their respective valence shells and achieving the stable electron configuration of helium. Because both hydrogen atoms have identical electronegativities, the shared electron pair is distributed evenly between them, resulting in a symmetrical and nonpolar molecule.
This nonpolar nature is crucial to understanding the properties of hydrogen gas. The absence of a dipole moment means that H₂ molecules do not exhibit strong intermolecular attractions. This explains why hydrogen gas exists as a diatomic molecule at room temperature and is relatively inert compared to many other molecules. The weak intermolecular forces lead to a low boiling point and low melting point.
Visualizing the Bond: Molecular Orbital Theory
A more sophisticated understanding of the hydrogen-hydrogen bond requires delving into molecular orbital theory. This theory describes how atomic orbitals combine to form molecular orbitals, which encompass the entire molecule. In H₂, the 1s atomic orbitals of each hydrogen atom overlap to form two molecular orbitals: a bonding molecular orbital and an antibonding molecular orbital.
The bonding molecular orbital is lower in energy than the original atomic orbitals, and the shared electron pair occupies this orbital. This electron pair is attracted to both nuclei, leading to the bond formation. The antibonding molecular orbital is higher in energy and remains unoccupied in the ground state of H₂. The energy difference between the bonding and antibonding orbitals determines the bond strength.
Comparing the H-H Bond to Other Covalent Bonds
To further appreciate the nature of the hydrogen-hydrogen bond, let's compare it to other covalent bonds:
-
H-O Bond (in water): This is a polar covalent bond due to the significant difference in electronegativity between hydrogen and oxygen. Oxygen attracts the shared electrons more strongly, leading to a partial negative charge on the oxygen and partial positive charges on the hydrogens. This polarity accounts for water's high boiling point and its exceptional solvent properties.
-
H-C Bond (in hydrocarbons): The electronegativity difference between hydrogen and carbon is relatively small, resulting in a slightly polar covalent bond. The H-C bond is less polar than the H-O bond, and hydrocarbons generally exhibit weaker intermolecular forces than water.
-
C-C Bond (in hydrocarbons): This is a nonpolar covalent bond due to the identical electronegativity of the carbon atoms. The strength of the C-C bond is considerable, making carbon chains very stable.
The comparison illustrates how the electronegativity difference between atoms significantly impacts the characteristics of the covalent bond and the resulting properties of the molecule.
The Importance of the Hydrogen-Hydrogen Bond
While seemingly simple, the hydrogen-hydrogen bond plays a crucial role in various contexts:
-
Energy Production: The combustion of hydrogen gas, which involves the breaking of H-H bonds and the formation of new bonds with oxygen, is a significant source of energy. Fuel cells also utilize the reaction between hydrogen and oxygen to generate electricity.
-
Industrial Processes: Hydrogen gas is a vital reactant in many industrial processes, including the synthesis of ammonia (Haber-Bosch process) and the refining of petroleum.
-
Biological Systems: While not directly involved in biological molecules in the same way as C-H or O-H bonds, the hydrogen-hydrogen bond is indirectly crucial. Hydrogen is a key component of water, the solvent of life. It’s also present in many biomolecules. Understanding hydrogen bonding (a different type of interaction) helps explain the properties of biological macromolecules like proteins and DNA.
-
Fundamental Research: Studying the hydrogen-hydrogen bond provides invaluable insights into the fundamental principles of chemical bonding and molecular structure. Its simplicity makes it an ideal model system for theoretical calculations and experimental studies.
Beyond the Basics: Isotopes and Bond Strength
The discussion so far has focused on the most common isotope of hydrogen, protium (¹H). However, deuterium (²H) and tritium (³H) are also isotopes of hydrogen, with different numbers of neutrons in their nuclei. This difference in mass slightly affects the vibrational frequency and bond strength of the H-H bond. Deuterium-deuterium (D₂) and tritium-tritium (T₂) bonds are slightly stronger than the protium-protium (H₂) bond, reflecting the increased mass and the associated changes in vibrational energy levels.
Advanced Concepts: Bond Length and Bond Energy
Several quantitative measures help characterize the hydrogen-hydrogen bond:
-
Bond length: This refers to the average distance between the two hydrogen nuclei in the H₂ molecule. The bond length is approximately 74 picometers (pm).
-
Bond energy: This is the amount of energy required to break the H-H bond and separate the two hydrogen atoms. The bond energy is approximately 436 kJ/mol. This value reflects the strength of the covalent bond.
Conclusion: A Foundation of Chemistry
The hydrogen-hydrogen bond, a simple yet powerful example of a nonpolar covalent bond, serves as a fundamental building block in chemistry. Understanding its formation, characteristics, and significance within a broader chemical context provides a strong foundation for exploring more complex chemical phenomena. From energy production to industrial processes and fundamental research, the role of this seemingly simple bond is immense and far-reaching. Its exploration continues to fuel advancements in various scientific disciplines, solidifying its importance as a cornerstone of chemical understanding. The simplicity of its structure allows for detailed theoretical analyses, constantly refining our understanding of chemical bonding itself. Further exploration into its isotopic variations and associated properties only enhances its significance within the greater field of chemistry.
Latest Posts
Latest Posts
-
The Manufacturing Overhead Account Is Debited When
Mar 19, 2025
-
Established A School For Navigation Science In Portugal
Mar 19, 2025
-
Which Symptoms Must Be Reported To A Manager
Mar 19, 2025
-
Lord Of The Flies Quotes With Page Numbers
Mar 19, 2025
-
Mendel Compnay Makes The Following Journal Entuty
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bond Is Joining The Two Hydrogen Atoms . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
