Where Are Metals Located On The Periodic Table
Breaking News Today
Mar 19, 2025 · 6 min read
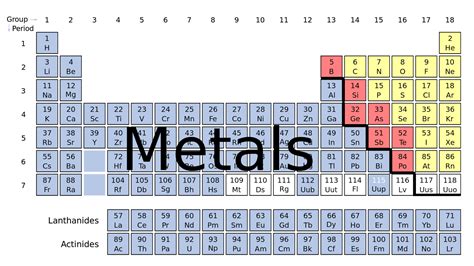
Table of Contents
Where Are Metals Located on the Periodic Table? A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding the arrangement of elements allows us to predict their behavior and reactivity. One of the most fundamental classifications within the periodic table is the distinction between metals, nonmetals, and metalloids. This article delves into the specific location of metals on the periodic table, exploring their characteristics and the exceptions that exist.
The Broad Location of Metals
Metals are overwhelmingly dominant on the periodic table. They occupy a vast majority of the table, primarily situated on the left-hand side and extending across the middle section. A simple, albeit imperfect, rule of thumb is that elements to the left of the staircase-like line separating metals from nonmetals are metals. This line, however, isn't a perfectly rigid boundary.
The Staircase Line: A Delicate Boundary
The staircase line, running from Boron (B) to Astatine (At), roughly separates metals from nonmetals. Elements directly bordering this line, known as metalloids or semimetals, exhibit properties of both metals and nonmetals. Their positioning highlights the gradual transition in properties between the two categories. The ambiguity around this line is a testament to the gradual nature of chemical properties within the periodic table.
Specific Groups and Periods Containing Metals
Let's examine the major groups and periods where metals reside:
Group 1: Alkali Metals
The alkali metals (lithium, sodium, potassium, rubidium, cesium, and francium) are highly reactive metals located in the first column (Group 1) of the periodic table. Their reactivity stems from their single valence electron, which they readily lose to form +1 ions. This characteristic makes them extremely reactive with water and air.
Group 2: Alkaline Earth Metals
Alkaline earth metals (beryllium, magnesium, calcium, strontium, barium, and radium) occupy the second column (Group 2). With two valence electrons, they are less reactive than alkali metals but still readily form +2 ions. These metals are essential for various biological processes and industrial applications.
Transition Metals
The d-block elements, commonly referred to as transition metals, dominate the central region of the periodic table. These metals are characterized by their partially filled d orbitals, leading to a wide range of oxidation states and complex ion formation. They are known for their variable valency, colorful compounds, and catalytic properties, making them crucial in various industrial processes and technological applications. Examples include iron, copper, nickel, and gold.
Inner Transition Metals (Lanthanides and Actinides)
Located separately at the bottom of the periodic table, the f-block elements consist of the lanthanides (rare earth elements) and actinides. These elements are characterized by their filling of the f orbitals and have similar chemical properties within their respective series. Many actinides are radioactive.
Post-Transition Metals
These metals, located between the transition metals and metalloids, demonstrate a blend of metallic and non-metallic properties. They tend to be less reactive than the alkali and alkaline earth metals but show greater variability in their oxidation states compared to the main group metals. Examples include aluminum, tin, and lead.
Understanding Metallic Properties and their Location
The location of metals on the periodic table directly correlates with their characteristic properties:
Conductivity
Metals are excellent conductors of heat and electricity. This property arises from the delocalized nature of their valence electrons, allowing for easy movement of charge. The higher the density of delocalized electrons, generally found in metals closer to the left and bottom of the periodic table, the higher the conductivity.
Malleability and Ductility
Metals are malleable (can be hammered into sheets) and ductile (can be drawn into wires). This is due to the ability of metal atoms to slide past each other without breaking the metallic bonds. This property is again related to electron delocalization and the strength of metallic bonding, which varies depending on the metal's position on the periodic table.
Luster
The shiny appearance, or luster, of metals is a result of their interaction with light. The free movement of electrons allows them to absorb and re-emit light at various wavelengths, contributing to their characteristic metallic sheen. The intensity of luster can vary among metals based on their electron configuration and other factors.
Density
Metallic density varies significantly across the periodic table. Generally, metals towards the bottom left tend to have higher densities due to their larger atomic mass and tighter packing of atoms. However, this is not an absolute rule, and other factors like crystal structure and bonding also influence density.
Exceptions and Anomalies
While the general trend is clear, some exceptions and anomalies exist:
-
Hydrogen: Although positioned in Group 1, hydrogen's behavior is closer to nonmetals under standard conditions. It exists as a diatomic gas and does not exhibit typical metallic properties.
-
Boron: Despite its location near the staircase line, boron is considered a metalloid and shares more non-metallic characteristics.
-
Silicon: Located next to boron, silicon is another metalloid that demonstrates a blend of metallic and non-metallic properties.
-
Astatine: As a radioactive element, it's challenging to fully characterize its properties, but it is often considered a metalloid.
-
Polonium: While technically a metal, polonium displays some properties that deviate from typical metallic behavior. Its radioactivity significantly influences its characteristics.
These exceptions remind us that the periodic table's organization is a model, and some elements might show characteristics that blur the lines between classifications.
Applications and Importance of Metals
Metals' widespread application in various fields is directly linked to their unique properties. Their conductivity makes them essential in electrical wiring and electronics. Their strength and durability are crucial in construction, automotive, and aerospace industries. Their reactivity plays a vital role in chemical processes and catalysis. Transition metals, in particular, are indispensable in various catalytic converters and industrial processes. The use of metals is practically ubiquitous in modern life, from the structural components of buildings to the intricate circuitry within our smartphones.
Conclusion: A Dynamic Landscape
The location of metals on the periodic table is not merely a matter of arbitrary placement; it reflects the fundamental relationships between atomic structure, electronic configuration, and observable properties. While a simplified view places metals predominantly on the left and center, understanding the nuanced behavior of elements around the staircase line and the exceptions to general rules enhances our comprehension of the periodic table’s complexity. The periodic table serves as a powerful predictive tool, enabling us to anticipate the behavior of elements and exploit their unique properties for technological advancement and scientific discovery. Continued research and refinement of our understanding of atomic structure and chemical bonding will further illuminate the fascinating world of metallic elements and their strategic locations within the periodic table's intricate arrangement.
Latest Posts
Latest Posts
-
What Is The Most Abundant Gas In The Atmosphere
Mar 19, 2025
-
Raw Shell Eggs Must Be Received At What Temperature
Mar 19, 2025
-
Discuss The Role Of Behavior In Physical Fitness Levels
Mar 19, 2025
-
What Is The Major Product Of The Following Reaction
Mar 19, 2025
-
What Symptom Must Be Reported To A Manager
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Where Are Metals Located On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
