Where Are Nonmetals Located On The Periodic Table
Breaking News Today
Mar 17, 2025 · 5 min read
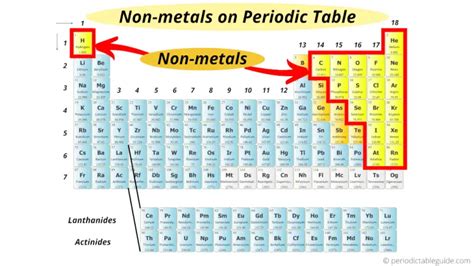
Table of Contents
Where Are Nonmetals Located on the Periodic Table? A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding the arrangement of elements allows us to predict their behavior and reactivity. One key aspect of this organization is the distinction between metals, nonmetals, and metalloids. This article will delve deep into the location of nonmetals on the periodic table, exploring their characteristics, properties, and exceptions.
Understanding the Periodic Table's Structure
Before pinpointing the location of nonmetals, let's briefly review the periodic table's structure. The table is arranged in rows called periods and columns called groups or families. Periods represent increasing energy levels of electrons, while groups reflect similar outer electron configurations, leading to similar chemical behaviors.
The table is broadly divided into three categories:
-
Metals: Typically located on the left side of the table. They are characterized by their good electrical and thermal conductivity, malleability (ability to be hammered into shapes), ductility (ability to be drawn into wires), and metallic luster (shiny appearance).
-
Nonmetals: Generally found on the right side of the table. These elements lack the typical metallic properties and often exhibit diverse chemical behaviors.
-
Metalloids (Semimetals): Positioned along a zigzag line separating metals and nonmetals. They display properties intermediate between metals and nonmetals, exhibiting characteristics of both.
Locating Nonmetals on the Periodic Table
Nonmetals are situated primarily in the upper right-hand corner of the periodic table. They occupy groups 14 (Carbon group) through 18 (Noble gases), excluding the elements bordering the metalloid staircase. This staircase, a diagonal line running from Boron (B) to Astatine (At), separates the metals from the nonmetals. Elements along this line are metalloids, exhibiting a mix of metallic and nonmetallic properties.
Specifically, the nonmetals include:
-
Group 14 (Carbon Group): Carbon (C), Silicon (Si) – though silicon is a metalloid – and Germanium (Ge) - a metalloid - show some nonmetallic character. Carbon, however, is distinctly a nonmetal.
-
Group 15 (Pnictogens): Nitrogen (N), Phosphorus (P), Arsenic (As) - a metalloid -, Antimony (Sb) - a metalloid -, and Bismuth (Bi) - a metalloid -. Nitrogen and Phosphorus are clearly nonmetals; the others increasingly exhibit metallic properties.
-
Group 16 (Chalcogens): Oxygen (O), Sulfur (S), Selenium (Se), Tellurium (Te), and Polonium (Po). Oxygen, Sulfur, and Selenium are distinctly nonmetals, while Tellurium and Polonium exhibit more metallic character.
-
Group 17 (Halogens): Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At). All halogens are nonmetals, although astatine's properties are less well-known due to its radioactivity and short half-life.
-
Group 18 (Noble Gases): Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn), and Oganesson (Og). These elements are all nonmetals and are known for their extremely low reactivity.
It is crucial to note the gradual transition from nonmetallic to metallic character as you move down a group or from right to left across a period. This transition is reflected in the properties of the elements and their placement near the metalloid boundary.
Properties of Nonmetals
Nonmetals demonstrate a diverse range of properties that distinguish them from their metallic counterparts. Some key characteristics include:
-
Poor electrical and thermal conductivity: Nonmetals are generally poor conductors of electricity and heat. This is due to their electron configuration; they don't have free-flowing electrons like metals.
-
Brittle: Unlike the malleable metals, nonmetals are often brittle and tend to shatter when struck.
-
Lack of metallic luster: Nonmetals typically lack the shiny, metallic appearance characteristic of metals. They can appear dull or have various colors.
-
Low density: Nonmetals generally have lower densities compared to metals.
-
Varied states of matter: Nonmetals exist in all three common states of matter at room temperature: solid (e.g., carbon, sulfur), liquid (e.g., bromine), and gas (e.g., oxygen, nitrogen).
-
High electronegativity: Nonmetals generally have high electronegativity, meaning they strongly attract electrons in a chemical bond. This leads to their tendency to form covalent bonds.
-
Form anions: Nonmetals tend to gain electrons to achieve a stable electron configuration, forming negatively charged ions called anions.
Exceptions and Ambiguity
While the general location of nonmetals is clear, some exceptions and ambiguities exist, especially near the metalloid boundary. The classification of elements as metals, nonmetals, or metalloids can sometimes be subjective and depend on the specific properties being considered. For instance, some elements may exhibit metallic characteristics under certain conditions but behave as nonmetals under others.
Hydrogen (H), located in group 1, is a unique case. It is often placed above the alkali metals, but its properties are distinctly nonmetallic under standard conditions, behaving more like a nonmetal than a metal. However, under high pressure, it can exhibit metallic properties.
Silicon (Si) and Germanium (Ge), located in group 14, border the metalloid line and show some characteristics of both metals and nonmetals. They are typically classified as metalloids, demonstrating semiconductor properties.
Importance of Understanding Nonmetal Location
Understanding the location and properties of nonmetals on the periodic table is crucial for several reasons:
-
Predicting Chemical Behavior: The periodic table provides a framework for predicting how elements will interact. Knowing that an element is a nonmetal helps anticipate its reactivity, bonding behavior, and the types of compounds it will form.
-
Material Science: The properties of nonmetals are vital in material science and engineering. Nonmetallic materials have diverse applications, ranging from plastics and ceramics to semiconductors and insulators.
-
Biological Systems: Many essential biological molecules, including proteins, nucleic acids, and carbohydrates, are composed of nonmetals like carbon, oxygen, nitrogen, and phosphorus. Understanding the chemistry of these elements is critical in biological research and medicine.
-
Industrial Applications: Nonmetals are critical components in numerous industrial processes. For example, chlorine is used in water purification, nitrogen in fertilizers, and oxygen in various combustion processes.
Conclusion
The location of nonmetals on the periodic table is primarily in the upper right-hand corner, though with exceptions near the metalloid boundary. Understanding their position and the gradual transition to metallic properties helps us predict their behaviors and appreciate their vital roles in diverse scientific fields, from chemistry and material science to biology and industry. While classifications can sometimes be nuanced, the periodic table provides a fundamental framework for comprehending the relationship between an element's location and its properties. Further study of the periodic table and the properties of individual elements will enhance your understanding of this crucial organizing principle in chemistry.
Latest Posts
Latest Posts
-
Cdl Combination Test Questions And Answers Pdf
Mar 18, 2025
-
Life Insurance Exam Questions And Answers Pdf
Mar 18, 2025
-
The Direct Carry Is Used To Transfer A Patient
Mar 18, 2025
-
The Emancipation Proclamation Of January 1 1863 Quizlet
Mar 18, 2025
-
These Cards Will Get You Drunk Quizlet
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Where Are Nonmetals Located On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
