Which Of The Following Is Not A Greenhouse Gas
Breaking News Today
Apr 03, 2025 · 5 min read
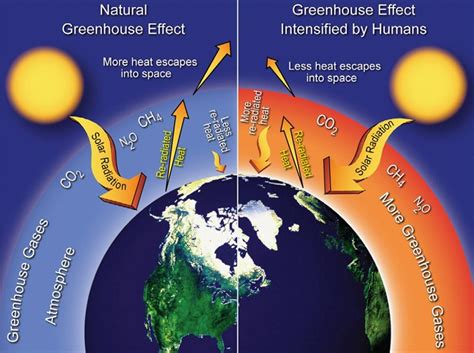
Table of Contents
- Which Of The Following Is Not A Greenhouse Gas
- Table of Contents
- Which of the Following is NOT a Greenhouse Gas? Understanding Atmospheric Composition
- Defining Greenhouse Gases
- The Major Greenhouse Gases
- Gases That Are NOT Greenhouse Gases
- The Importance of Understanding Atmospheric Composition
- Beyond the Basics: Factors Influencing Greenhouse Gas Effects
- Conclusion: The Role of Non-Greenhouse Gases
- Latest Posts
- Latest Posts
- Related Post
Which of the Following is NOT a Greenhouse Gas? Understanding Atmospheric Composition
The Earth's atmosphere is a complex mixture of gases, each playing a vital role in shaping our planet's climate and habitability. A critical aspect of this atmospheric composition is the presence of greenhouse gases (GHGs). These gases trap heat within the atmosphere, contributing to the greenhouse effect, a natural process that keeps our planet warm enough to support life. However, human activities have significantly increased the concentration of certain GHGs, leading to an enhanced greenhouse effect and climate change. Understanding which gases contribute to this effect, and which do not, is crucial to comprehending and addressing climate change.
Defining Greenhouse Gases
Before we delve into identifying which gas is not a greenhouse gas, let's first define what constitutes a greenhouse gas. Greenhouse gases are gases that absorb and emit infrared radiation (heat) within the thermal infrared range. This absorption and emission process traps heat within the atmosphere, preventing it from escaping into space. The key characteristic is their ability to interact with infrared radiation, a property determined by their molecular structure. Molecules with three or more atoms, particularly those with asymmetric structures, are most effective at absorbing infrared radiation.
The Major Greenhouse Gases
Several gases contribute significantly to the greenhouse effect. These include:
-
Water Vapor (H₂O): This is the most abundant greenhouse gas in the atmosphere. While naturally occurring, its concentration is influenced by temperature, creating a feedback loop in the climate system. Warmer temperatures lead to more evaporation and increased water vapor, further enhancing the greenhouse effect.
-
Carbon Dioxide (CO₂): Released through natural processes like respiration and volcanic eruptions, CO₂ concentrations have dramatically increased due to human activities, primarily the burning of fossil fuels (coal, oil, and natural gas) and deforestation. CO₂ is a particularly potent GHG, remaining in the atmosphere for centuries.
-
Methane (CH₄): A much more potent greenhouse gas than CO₂, methane is emitted from various sources, including livestock agriculture, natural gas leaks, rice cultivation, and landfills. While it has a shorter atmospheric lifetime than CO₂, its high warming potential makes it a significant contributor to climate change.
-
Nitrous Oxide (N₂O): Also known as laughing gas, N₂O is released through agricultural practices, industrial processes, and the burning of fossil fuels. It's a very potent greenhouse gas with a long atmospheric lifetime.
-
Ozone (O₃): Ozone in the stratosphere protects us from harmful ultraviolet radiation. However, ground-level ozone is a potent pollutant and greenhouse gas, formed through chemical reactions involving pollutants from vehicles and industrial emissions.
-
Fluorinated Gases: These are synthetic gases used in various industrial applications, including refrigeration and air conditioning. They are exceptionally potent greenhouse gases, with extremely long atmospheric lifetimes. Examples include hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), sulfur hexafluoride (SF₆), and nitrogen trifluoride (NF₃).
Gases That Are NOT Greenhouse Gases
Now, let's address the main question: which gases are not considered greenhouse gases? Several gases in the atmosphere are largely transparent to infrared radiation and, therefore, do not significantly contribute to the greenhouse effect. These include:
-
Nitrogen (N₂): The most abundant gas in the Earth's atmosphere (about 78%), nitrogen is a diatomic molecule (N=N). Its symmetrical structure prevents it from effectively absorbing and emitting infrared radiation. Therefore, it does not contribute significantly to the greenhouse effect.
-
Oxygen (O₂): The second most abundant gas in the atmosphere (about 21%), oxygen, like nitrogen, is a diatomic molecule (O=O). Its symmetrical structure means it's largely transparent to infrared radiation, making it a non-greenhouse gas.
-
Argon (Ar): A noble gas, argon is a monatomic gas and does not interact with infrared radiation. It plays no significant role in the greenhouse effect.
-
Neon (Ne), Helium (He), Krypton (Kr), Xenon (Xe): These are other noble gases present in trace amounts in the atmosphere. Like argon, they are monatomic and do not contribute to the greenhouse effect.
The Importance of Understanding Atmospheric Composition
Understanding the composition of the Earth's atmosphere, specifically identifying which gases contribute to the greenhouse effect and which do not, is vital for several reasons:
-
Climate Change Mitigation: Accurately identifying and quantifying greenhouse gas emissions is crucial for developing effective strategies to mitigate climate change. Policies and regulations aimed at reducing GHG emissions are based on this understanding.
-
Atmospheric Modeling: Accurate climate models require a comprehensive understanding of atmospheric composition and the radiative properties of various gases. These models help us predict future climate scenarios and assess the impacts of climate change.
-
Environmental Monitoring: Monitoring atmospheric concentrations of greenhouse gases helps scientists track changes over time and assess the effectiveness of mitigation efforts.
-
Public Awareness: Educating the public about the science behind climate change, including the role of greenhouse gases, is crucial for fostering public support for climate action.
Beyond the Basics: Factors Influencing Greenhouse Gas Effects
The impact of a greenhouse gas is not solely determined by its presence in the atmosphere. Several other factors play a crucial role:
-
Atmospheric Lifetime: The time a gas remains in the atmosphere influences its overall warming effect. Gases with longer lifetimes have a more sustained impact on climate change.
-
Global Warming Potential (GWP): GWP is a measure of how much heat a gas traps in the atmosphere over a specific time period compared to carbon dioxide (CO₂), which is used as a reference point (GWP=1). Methane, for instance, has a much higher GWP than CO₂ over shorter timeframes, but its shorter atmospheric lifetime means its overall impact is less over longer periods.
-
Concentration: The abundance of a gas in the atmosphere directly affects its contribution to the greenhouse effect. Even a relatively weak greenhouse gas can have a significant impact if its concentration is high.
Conclusion: The Role of Non-Greenhouse Gases
While greenhouse gases are the primary drivers of the Earth's climate system and the current climate crisis, it's crucial to acknowledge the roles of gases that do not contribute to the greenhouse effect. These non-greenhouse gases, predominantly nitrogen and oxygen, constitute the majority of the Earth's atmosphere. Understanding their properties and their interactions with the greenhouse gases is essential for accurately modeling and predicting climate change and devising effective mitigation strategies. The complex interplay of all atmospheric constituents underscores the need for continued research and monitoring to fully comprehend our planet's climate and ensure its future sustainability. Focusing solely on GHGs without considering the broader atmospheric context would provide an incomplete and potentially misleading picture of the climate system.
Latest Posts
Latest Posts
-
You May Cross A Double Solid Yellow Line
Apr 06, 2025
-
What Are The Recommended Training Variables For Self Myofascial Rolling
Apr 06, 2025
-
America The Story Of Us Episode 2 Revolution
Apr 06, 2025
-
The Cost Of Direct Materials Are Classified As
Apr 06, 2025
-
Which Of These Sentences Is An Example Of Paradox
Apr 06, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is Not A Greenhouse Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
