Which Statement Describes How Enzymes And Substrates Are Related
Breaking News Today
Mar 17, 2025 · 6 min read
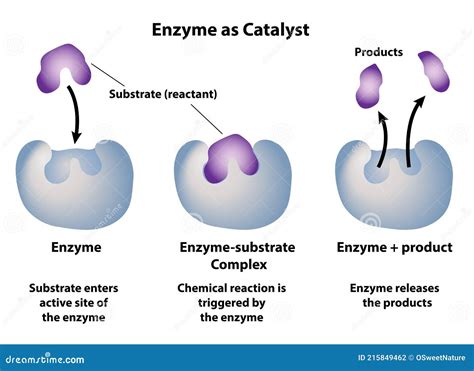
Table of Contents
Which Statement Describes How Enzymes and Substrates are Related? A Deep Dive into Enzyme-Substrate Interactions
Enzymes are biological catalysts, vital for virtually every biochemical reaction within living organisms. Their function hinges on their interaction with specific molecules called substrates. Understanding the relationship between enzymes and substrates is fundamental to comprehending the intricacies of life itself. This article will explore this relationship in detail, examining the key aspects of enzyme-substrate interactions, including the lock-and-key model, the induced fit model, factors influencing enzyme activity, and the significance of this relationship in various biological processes.
The Fundamental Relationship: Enzymes and Their Substrates
The relationship between an enzyme and its substrate can be summarized as follows: enzymes are highly specific biological catalysts that bind to specific substrates, facilitating the conversion of substrates into products. This specificity arises from the precise three-dimensional structure of the enzyme, which determines the shape and chemical properties of its active site – the region where the substrate binds.
Specificity: The Key to Enzyme Function
Enzyme specificity is a crucial aspect of their function. It means that each enzyme typically catalyzes only one or a very limited range of reactions involving specific substrates. This high degree of specificity is essential for the precise regulation of metabolic pathways within cells. Different enzymes have different active sites, like different keys fitting different locks. This specificity ensures that the right reactions occur at the right time and place within a cell.
Factors contributing to enzyme specificity:
- Shape and size complementarity: The active site's shape and size must complement that of the substrate. This is crucial for the formation of the enzyme-substrate complex.
- Electrostatic interactions: Charged amino acid residues within the active site interact with complementary charges on the substrate, strengthening the binding.
- Hydrogen bonds: Hydrogen bonds between specific atoms on the enzyme and substrate contribute to binding affinity and specificity.
- Hydrophobic interactions: Non-polar regions on the enzyme and substrate can interact favorably, further enhancing binding.
Models Explaining Enzyme-Substrate Interaction
Two primary models attempt to explain how enzymes and substrates interact:
1. The Lock-and-Key Model
The lock-and-key model, proposed by Emil Fischer in 1894, envisions the enzyme's active site as a rigid, pre-formed structure that perfectly complements the substrate's shape, like a lock and key. The substrate fits precisely into the active site, forming the enzyme-substrate complex. This complex then undergoes a transition state, leading to the formation of products, after which the products are released, and the enzyme returns to its original state.
Limitations of the Lock-and-Key Model:
This model, while conceptually simple, has limitations. It doesn't adequately explain how enzymes can accommodate substrates with slightly different structures or how they can catalyze reactions involving significant structural changes in the substrate.
2. The Induced Fit Model
The induced fit model, proposed by Daniel Koshland in 1958, provides a more nuanced and accurate description of enzyme-substrate interactions. This model suggests that the enzyme's active site is flexible and undergoes conformational changes upon substrate binding. The substrate's binding induces a change in the enzyme's shape, optimizing the active site for catalysis. This induced fit enhances the binding affinity and promotes the transition state, leading to efficient catalysis.
Advantages of the Induced Fit Model:
The induced fit model better explains the following observations:
- Enzyme flexibility: It accounts for the flexibility of enzymes and their ability to adapt to different substrates.
- Substrate-induced conformational changes: It explains how substrate binding can induce conformational changes that optimize catalysis.
- Broader substrate specificity: It allows for the possibility of enzymes interacting with a broader range of substrates than predicted by the lock-and-key model.
Factors Affecting Enzyme-Substrate Interactions
Several factors influence the rate and efficiency of enzyme-substrate interactions:
1. Substrate Concentration
At low substrate concentrations, the reaction rate increases proportionally with substrate concentration. This is because more substrate molecules are available to bind to the available enzyme molecules. However, at high substrate concentrations, the reaction rate plateaus. This is because all enzyme active sites are saturated with substrate, and further increases in substrate concentration do not increase the reaction rate significantly. This saturation point is represented by the maximum velocity (Vmax) of the reaction.
2. Enzyme Concentration
Increasing enzyme concentration increases the rate of the reaction, provided that there is sufficient substrate available. More enzyme molecules mean more active sites are available to bind substrates and catalyze the reaction.
3. Temperature
Enzymes have an optimal temperature at which they function most efficiently. Increasing temperature generally increases the rate of enzyme-catalyzed reactions, as it increases the kinetic energy of molecules, leading to more frequent collisions between enzyme and substrate. However, excessively high temperatures can denature the enzyme, leading to loss of its activity. Denaturation disrupts the enzyme's three-dimensional structure, rendering its active site non-functional.
4. pH
Enzymes also have an optimal pH at which they function best. Changes in pH can affect the enzyme's ionization state, altering the charge distribution in the active site and potentially disrupting enzyme-substrate interactions. Extreme pH values can also denature the enzyme.
5. Inhibitors
Enzyme inhibitors are molecules that can decrease the rate of enzyme-catalyzed reactions. They can bind to the enzyme's active site, directly blocking substrate binding (competitive inhibition), or bind to other sites on the enzyme, causing conformational changes that reduce the enzyme's activity (non-competitive inhibition).
The Significance of Enzyme-Substrate Interactions in Biological Processes
Enzyme-substrate interactions are crucial for a wide range of biological processes, including:
1. Metabolism:
Enzymes are central to metabolic pathways, facilitating the breakdown of nutrients (catabolism) and the synthesis of essential molecules (anabolism). Without these highly specific enzyme-substrate interactions, these pathways would be inefficient or non-existent. For example, the digestive enzymes in our bodies break down food molecules into smaller components for absorption.
2. DNA Replication and Repair:
Enzymes play a vital role in DNA replication and repair. DNA polymerases, for example, are enzymes that synthesize new DNA strands by adding nucleotides to the growing chain. Their ability to selectively add the correct nucleotides is dependent on highly specific interactions with the DNA template and the incoming nucleotides.
3. Protein Synthesis:
Ribosomes, complex molecular machines responsible for protein synthesis, rely on specific interactions between tRNA molecules (carrying amino acids) and mRNA (carrying the genetic code). These interactions, guided by enzymes, ensure the accurate translation of genetic information into proteins.
4. Signal Transduction:
Enzymes are involved in signal transduction pathways, relaying signals from the cell surface to the interior. These pathways often involve cascades of enzymatic reactions, where the product of one enzyme becomes the substrate for another.
5. Cellular Regulation:
Enzyme activity is tightly regulated to maintain cellular homeostasis. This regulation involves mechanisms like feedback inhibition, where the product of a metabolic pathway inhibits an earlier enzyme in the pathway, preventing overproduction.
Conclusion: Understanding the Enzyme-Substrate Relationship
The relationship between enzymes and substrates is a cornerstone of biochemistry. The high specificity of enzymes, facilitated by the unique three-dimensional structure of their active sites, is crucial for the efficient and precise catalysis of biological reactions. While the lock-and-key model provides a simplified understanding, the induced fit model more accurately reflects the dynamic nature of enzyme-substrate interactions. Understanding these interactions is essential for comprehending the intricate workings of life, from metabolic processes to cellular regulation, and for developing therapeutic interventions targeting specific enzymes involved in disease. Further research continues to refine our understanding of this fundamental biological interaction, opening new avenues for advancements in medicine, biotechnology, and other fields.
Latest Posts
Latest Posts
-
If An Individual Is Heterozygous For A Particular Trait
Mar 18, 2025
-
If You Add More Enzyme The Reaction Will
Mar 18, 2025
-
The Purpose Of A Hazcom Program Is To Ensure That
Mar 18, 2025
-
Describe The Continuous Nature Of The Physical Fitness Concept
Mar 18, 2025
-
High Levels Of Cholesterol Can First Lead Directly To
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Statement Describes How Enzymes And Substrates Are Related . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
