Why Is Water Considered A Polar Molecule
Breaking News Today
Mar 19, 2025 · 6 min read
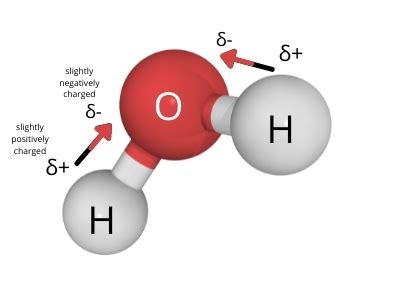
Table of Contents
Why Is Water Considered a Polar Molecule? Understanding the Science Behind Water's Unique Properties
Water. The lifeblood of our planet, the solvent of life, the ubiquitous substance shaping our world. But what makes water so special? Why does it possess such unique properties, from its high boiling point to its ability to dissolve a vast array of substances? The answer lies in its molecular structure and the resulting polarity. This article delves deep into the reasons why water is considered a polar molecule, exploring the concepts of electronegativity, bond polarity, and molecular geometry, and demonstrating how these factors contribute to water's remarkable characteristics.
Understanding Polarity: A Tale of Unequal Sharing
At the heart of water's polarity lies the concept of polarity, a measure of how equally electrons are shared between atoms in a chemical bond. In a perfectly nonpolar bond, like the one in a diatomic oxygen molecule (O₂), electrons are shared equally between the two identical oxygen atoms. However, in many molecules, electrons are drawn more strongly to one atom than another, creating a polar covalent bond. This unequal sharing of electrons leads to a difference in charge distribution within the molecule, resulting in a dipole moment.
Electronegativity: The Tug-of-War of Electrons
Electronegativity is the measure of an atom's ability to attract electrons towards itself in a chemical bond. Different elements exhibit different electronegativities. Oxygen, with its high electronegativity, strongly attracts electrons. Hydrogen, with its relatively low electronegativity, has a weaker pull on electrons.
The Water Molecule: An Unequal Partnership
A water molecule (H₂O) consists of one oxygen atom covalently bonded to two hydrogen atoms. Due to oxygen's higher electronegativity, the shared electrons in the O-H bonds spend more time closer to the oxygen atom than to the hydrogen atoms. This creates a partial negative charge (δ-) on the oxygen atom and partial positive charges (δ+) on the hydrogen atoms. This uneven distribution of charge is what makes water a polar molecule.
Molecular Geometry: The Shape Matters
The molecular geometry, or the three-dimensional arrangement of atoms in a molecule, also plays a crucial role in determining polarity. While the individual O-H bonds are polar, the overall polarity of the molecule depends on the arrangement of these bonds. Water adopts a bent or V-shaped molecular geometry due to the presence of two lone pairs of electrons on the oxygen atom. These lone pairs repel the bonding pairs, causing the H-O-H bond angle to be approximately 104.5 degrees, rather than the 180 degrees expected in a linear molecule.
The Impact of Bent Geometry on Polarity
This bent shape is critical because it prevents the individual bond dipoles from canceling each other out. If the water molecule were linear, the two O-H bond dipoles would point in opposite directions and cancel each other, resulting in a nonpolar molecule. However, the bent geometry ensures that the bond dipoles add up vectorially, resulting in a net dipole moment, confirming water's polar nature.
Consequences of Water's Polarity: A Cascade of Unique Properties
The polarity of water is not just an abstract concept; it's the root cause of many of its exceptional properties that are essential for life on Earth.
1. High Boiling Point and Surface Tension: Hydrogen Bonding
Water molecules are capable of forming hydrogen bonds, a strong type of intermolecular force. A hydrogen bond is a special type of dipole-dipole attraction that occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a nearby molecule. In water, the partially positive hydrogen atoms of one molecule are attracted to the partially negative oxygen atoms of neighboring molecules.
These hydrogen bonds are responsible for water's unusually high boiling point and surface tension. To boil water, enough energy must be supplied to overcome the relatively strong hydrogen bonds holding the molecules together. Similarly, surface tension results from the strong cohesive forces between water molecules due to hydrogen bonding.
2. Excellent Solvent: Dissolving Power
Water's polarity makes it an exceptional solvent, capable of dissolving a wide range of ionic and polar substances. When an ionic compound like sodium chloride (NaCl) is added to water, the partially negative oxygen atoms of water molecules surround the positively charged sodium ions (Na⁺), while the partially positive hydrogen atoms surround the negatively charged chloride ions (Cl⁻). This process, known as hydration, effectively breaks down the ionic lattice and dissolves the salt. Similarly, polar molecules can dissolve in water because of the dipole-dipole interactions between the solute and solvent molecules.
3. High Specific Heat Capacity: Temperature Moderation
Water has a high specific heat capacity, meaning it can absorb a large amount of heat energy with a relatively small temperature change. This is due to the strong hydrogen bonds between water molecules. A significant amount of energy is required to break these bonds and increase the kinetic energy of the molecules, leading to a smaller temperature increase. This property is crucial for regulating Earth's temperature and maintaining stable climates.
4. Cohesion and Adhesion: Water Transport in Plants
Water molecules exhibit strong cohesion (attraction to each other) and adhesion (attraction to other surfaces) due to hydrogen bonding. These properties are essential for the transport of water in plants through capillary action. Water molecules adhere to the walls of the xylem vessels, and their cohesive forces pull other water molecules upward, allowing water to be transported against gravity from the roots to the leaves.
Beyond the Basics: Exploring Further Aspects of Water's Polarity
The polarity of water is a fundamental concept with far-reaching implications. Understanding its influence on various properties allows us to grasp the significance of water in numerous natural processes and technological applications.
Water's Role in Biological Systems: A Polar World
Water's polarity is central to the functioning of biological systems. It acts as a medium for biochemical reactions, participates in metabolic processes, and plays a crucial role in maintaining the structure and function of cells. The polar nature of biological molecules, such as proteins and DNA, is intimately linked to their interactions with water.
Applications of Water's Polarity: From Industry to Medicine
The unique properties of water stemming from its polarity are exploited extensively in various industries. Its excellent solvent properties are used in cleaning, industrial processes, and in the pharmaceutical industry. Its high specific heat capacity is utilized in cooling systems and temperature regulation. The cohesive and adhesive forces are harnessed in processes like inkjet printing and transpiration in plants.
Conclusion: The Polarity of Water – A Foundation for Life and Beyond
The polarity of water, stemming from its molecular structure and the unequal sharing of electrons between oxygen and hydrogen atoms, is the cornerstone of its remarkable properties. This seemingly simple molecule, with its bent geometry and potent hydrogen bonding, plays a pivotal role in shaping our planet and enabling life as we know it. Understanding water's polarity is fundamental to comprehending a vast array of natural phenomena and technological applications, solidifying its position as one of the most crucial and fascinating molecules in the universe. From the smallest biological processes to the largest climatic systems, the influence of water's polarity is undeniable and profound.
Latest Posts
Latest Posts
-
The Crossover Point Is That Production Quantity Where
Mar 19, 2025
-
Permanent Colors Containing Para Dyes Would Fall Into Which Color Category
Mar 19, 2025
-
Identify One Social Factor That Influenced American Imperialism
Mar 19, 2025
-
The Court Discovered Right To Implict In The Shawdows
Mar 19, 2025
-
When Does The Engage Stage Of The Inbound Methodology Begin
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Why Is Water Considered A Polar Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
