Why Is Water Considered A Polar Molecule Quizlet
Breaking News Today
Mar 23, 2025 · 6 min read
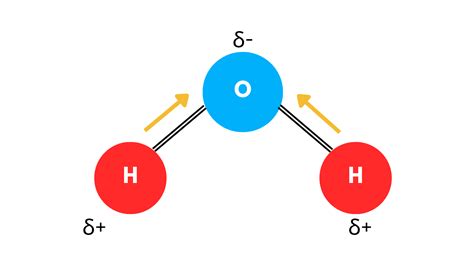
Table of Contents
Why is Water Considered a Polar Molecule? A Deep Dive
Water. It's the lifeblood of our planet, essential for all known forms of life. But beyond its biological importance, water possesses unique physical and chemical properties that stem from its molecular structure: it's a polar molecule. This seemingly simple fact underpins a vast array of its crucial characteristics. Understanding why water is polar is key to understanding its behavior and its critical role in the world around us. This comprehensive article will delve into the intricacies of water's polarity, exploring the underlying reasons and the significant consequences.
The Structure of a Water Molecule: The Foundation of Polarity
At the heart of water's polarity lies its molecular structure. A single water molecule (H₂O) consists of one oxygen atom covalently bonded to two hydrogen atoms. This isn't a symmetrical arrangement, however. The oxygen atom is significantly more electronegative than the hydrogen atoms.
Electronegativity: The Tug-of-War for Electrons
Electronegativity refers to an atom's ability to attract electrons in a chemical bond. Oxygen, being more electronegative than hydrogen, exerts a stronger pull on the shared electrons in the covalent bonds. This unequal sharing of electrons is crucial. It doesn't result in a complete transfer of electrons (which would form an ionic bond), but rather a polar covalent bond.
Bent Molecular Geometry: Amplifying the Polarity
The arrangement of atoms within the water molecule isn't linear; it's bent. This bent shape, with a bond angle of approximately 104.5 degrees, is a consequence of the presence of two lone pairs of electrons on the oxygen atom. These lone pairs repel the bonding pairs of electrons, pushing the hydrogen atoms closer together and resulting in the characteristic bent structure.
This bent geometry is critical because it ensures that the partial negative charge on the oxygen atom and the partial positive charges on the hydrogen atoms do not cancel each other out. Instead, they create a dipole moment, a measure of the molecule's overall polarity.
Understanding Partial Charges and the Dipole Moment
The unequal sharing of electrons in the polar covalent bonds leads to a partial negative charge (δ-) on the more electronegative oxygen atom and partial positive charges (δ+) on the less electronegative hydrogen atoms. These are not full charges like in an ionic compound, but rather represent a shift in electron density.
The dipole moment, represented by a vector pointing from the positive end to the negative end of the molecule, is a quantitative measure of this polarity. Water's significant dipole moment is a direct consequence of its bent geometry and the difference in electronegativity between oxygen and hydrogen.
The Consequences of Water's Polarity: A Cascade of Important Properties
The polarity of water is not merely an interesting molecular detail; it's the foundation for many of water's unique and vital properties. These properties are essential for life as we know it.
1. High Surface Tension: Water's Cohesive Forces
Water molecules are strongly attracted to each other due to hydrogen bonding. Hydrogen bonding is a special type of intermolecular force that occurs between a hydrogen atom bonded to a highly electronegative atom (like oxygen) and another electronegative atom in a separate molecule. This attraction, stemming directly from water's polarity, is responsible for water's remarkably high surface tension. This allows insects to walk on water and contributes to the capillary action that moves water up plant stems.
2. Excellent Solvent: Dissolving Many Substances
Water's polarity makes it an excellent solvent for many ionic and polar substances. The partial positive charges on the hydrogen atoms attract negatively charged ions or the negative poles of polar molecules, while the partial negative charge on the oxygen atom attracts positively charged ions or the positive poles of polar molecules. This interaction helps to break down and dissolve these substances, making water a crucial medium for biological processes.
3. High Specific Heat Capacity: Temperature Regulation
Water has an exceptionally high specific heat capacity. This means it takes a significant amount of energy to raise the temperature of water. This is largely due to the strong hydrogen bonds between water molecules. A substantial amount of energy is needed to break these bonds before the water molecules can increase their kinetic energy and thus their temperature. This property is critical in regulating temperature, both in the environment and within living organisms.
4. High Heat of Vaporization: Evaporative Cooling
Water also has a high heat of vaporization, meaning it requires a large amount of energy to change from a liquid to a gas. This is again a consequence of the strong hydrogen bonds. This property is crucial for evaporative cooling, a process that helps regulate temperature in organisms and environments. Sweating, for example, relies on water's high heat of vaporization to cool the body.
5. Density Anomaly: Ice Floats
Water exhibits a unique density anomaly. Ice, the solid form of water, is less dense than liquid water. This is because the hydrogen bonds in ice create a more open, crystalline structure compared to liquid water. This seemingly simple property has profound implications for aquatic life; ice floating on water insulates the water below, preventing it from freezing solid and allowing life to persist beneath the ice.
6. Universal Solvent: Supporting Life Processes
The term "universal solvent" highlights water's ability to dissolve a wide range of substances. This characteristic is fundamental to its role in biological systems. Water acts as a transport medium, carrying nutrients and waste products throughout organisms. It also serves as a reaction medium, providing a suitable environment for biochemical reactions to occur.
Beyond the Basics: Exploring Further Implications of Water's Polarity
The consequences of water's polarity extend far beyond the properties listed above. Its influence is seen in many aspects of chemistry, biology, geology, and even meteorology.
Water and Chemical Reactions: A Crucial Medium
Water's polar nature plays a crucial role in many chemical reactions. It acts as a reactant in some reactions (e.g., hydrolysis), a solvent for reactants, and it influences reaction rates through its ability to stabilize or destabilize charged intermediates.
Water and Biological Macromolecules: Structure and Function
The polarity of water has a profound impact on the structure and function of biological macromolecules like proteins and nucleic acids. The hydrophilic (water-loving) and hydrophobic (water-fearing) interactions between these molecules, influenced by their interactions with water, are crucial for their folding, stability, and function.
Water and Climate Regulation: A Global Perspective
Water's high specific heat capacity and heat of vaporization play critical roles in regulating global climate patterns. Large bodies of water, such as oceans, moderate temperature fluctuations, preventing extreme temperature swings. The water cycle, driven by water's evaporation and condensation, plays a key role in distributing heat around the planet.
Conclusion: The Unparalleled Importance of Water's Polarity
Water's polarity is not simply a scientific fact; it's a fundamental property that underlies its extraordinary importance for life and the planet's processes. From the microscopic level of molecular interactions to the macroscopic level of global climate patterns, the consequences of water's polarity are far-reaching and profound. Understanding this polarity is essential to understanding the world around us and the vital role water plays in sustaining life on Earth. The intricate interplay between water's molecular structure, its polarity, and its resulting properties paints a picture of a truly remarkable and essential substance. Further exploration into the multifaceted implications of water's polarity will continue to unveil its secrets and deepen our appreciation for its critical role in the functioning of our planet.
Latest Posts
Latest Posts
-
The Reasons That Nations Trade Includes The Fact That
Mar 25, 2025
-
To Ensure Their Availability For Worldwide Assignment
Mar 25, 2025
-
Period Costs For A Manufacturing Company Flow Directly To
Mar 25, 2025
-
What Is An Example Of An Operational Load Requirement
Mar 25, 2025
-
Which Event Must Precede All Others During Tissue Repair
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Why Is Water Considered A Polar Molecule Quizlet . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
