Cardiogenic Shock Following Ami Is Caused By Quizlet
Breaking News Today
Mar 17, 2025 · 8 min read
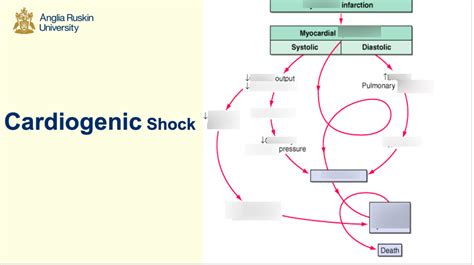
Table of Contents
Cardiogenic Shock Following AMI: Causes, Mechanisms, and Management
Cardiogenic shock (CS) following acute myocardial infarction (AMI) represents a severe complication with high mortality. Understanding its causes and mechanisms is crucial for timely intervention and improved patient outcomes. This comprehensive article will delve into the pathophysiology, risk factors, diagnostic approaches, and management strategies for cardiogenic shock post-AMI.
What is Cardiogenic Shock?
Cardiogenic shock is a life-threatening condition characterized by the heart's inability to pump enough oxygenated blood to meet the body's metabolic demands. Following an AMI, a significant portion of the heart muscle is damaged, impairing its contractile function. This leads to a reduction in cardiac output, causing a cascade of events that compromise vital organ perfusion. The consequences can be devastating, including multi-organ failure and death.
Causes of Cardiogenic Shock After AMI: A Deep Dive
The primary cause of cardiogenic shock post-AMI is extensive myocardial necrosis. This significant loss of functional heart muscle reduces the heart's ability to effectively pump blood. The extent of myocardial damage directly correlates with the risk of developing CS. Several factors contribute to the severity of myocardial injury and the subsequent development of cardiogenic shock:
-
Large infarct size: The larger the area of infarcted myocardium, the greater the reduction in cardiac contractility and the higher the likelihood of CS. This is often seen with anterior wall infarcts due to the larger mass of the left ventricle involved.
-
Left ventricular dysfunction: The left ventricle is the primary pump responsible for circulating oxygenated blood throughout the body. Significant damage to this chamber severely impairs its ability to pump effectively, directly leading to reduced cardiac output and CS. This dysfunction can manifest as reduced ejection fraction (EF), a key indicator of heart pump efficiency.
-
Right ventricular infarction: While less common, right ventricular infarction (RVI) can also lead to cardiogenic shock. The right ventricle plays a crucial role in returning blood to the lungs for oxygenation. Damage to the right ventricle can cause impaired venous return and decreased cardiac output. This is often associated with inferior wall myocardial infarctions.
-
Papillary muscle rupture: The papillary muscles are small muscles within the ventricles that support the mitral and tricuspid valves. Damage to these muscles, commonly due to AMI, can cause valve dysfunction, leading to mitral regurgitation (backflow of blood into the left atrium) and decreased cardiac output. This valve dysfunction significantly exacerbates the already compromised cardiac function.
-
Ventricular septal rupture: A rupture in the septum, the wall separating the left and right ventricles, allows blood to shunt from the left ventricle to the right, further reducing effective cardiac output and leading to cardiogenic shock. This is a catastrophic complication requiring immediate intervention.
-
Free wall rupture: This is a potentially fatal complication where a portion of the ventricular wall tears, causing massive internal bleeding and circulatory collapse.
-
Myocardial stunning: Even in areas not directly affected by infarction, myocardial stunning can occur. This temporary impairment of myocardial function can further reduce cardiac output and contribute to the development of CS. This is often reversible with appropriate treatment.
-
Pre-existing cardiac conditions: Patients with underlying heart conditions, such as pre-existing left ventricular dysfunction, coronary artery disease, or valvular heart disease, are at a significantly increased risk of developing cardiogenic shock following AMI. These pre-existing conditions weaken the heart's reserve capacity, making it less resilient to the added stress of an AMI.
Mechanisms of Cardiogenic Shock Post-AMI
The development of cardiogenic shock after AMI involves a complex interplay of several pathophysiological mechanisms:
-
Reduced Cardiac Output: The core problem is a significant decrease in the amount of blood pumped by the heart each minute. This reduction is a direct consequence of the damaged myocardium's inability to contract effectively.
-
Decreased Systemic Perfusion: The reduced cardiac output leads to inadequate blood flow to vital organs, including the brain, kidneys, and intestines. This results in tissue hypoxia (lack of oxygen) and cellular dysfunction.
-
Activation of the Sympathetic Nervous System: The body's response to low blood pressure and organ hypoperfusion involves the activation of the sympathetic nervous system. This leads to increased heart rate and peripheral vasoconstriction (narrowing of blood vessels), attempting to compensate for reduced cardiac output. However, this compensatory mechanism is often insufficient and can exacerbate myocardial oxygen demand.
-
Fluid Retention: The kidneys, in an attempt to increase blood volume, retain fluid. This, however, often leads to pulmonary edema (fluid accumulation in the lungs), worsening the patient's condition.
-
Metabolic Acidosis: Tissue hypoxia results in the accumulation of lactic acid, leading to metabolic acidosis. This further compromises cellular function and organ perfusion.
-
Inflammatory Response: Myocardial infarction triggers a significant inflammatory response, which can contribute to myocardial dysfunction and organ damage.
Diagnosis of Cardiogenic Shock Post-AMI
The diagnosis of cardiogenic shock post-AMI relies on a combination of clinical presentation, physical examination findings, and laboratory investigations:
-
Clinical Presentation: Patients typically present with signs and symptoms of hypoperfusion, including hypotension (low blood pressure), tachycardia (rapid heart rate), cool and clammy skin, altered mental status, and oliguria (reduced urine output). Chest pain may still be present or may have subsided.
-
Physical Examination: The physical examination often reveals signs of reduced peripheral perfusion, such as weak pulses and delayed capillary refill. Lung sounds may indicate pulmonary edema.
-
Electrocardiogram (ECG): ECG confirms the presence of AMI and assesses the extent of myocardial damage. ST-segment elevation or depression indicates the location and severity of the infarct.
-
Cardiac Enzymes: Elevated cardiac enzymes (troponin, CK-MB) confirm myocardial necrosis.
-
Echocardiography: Echocardiography provides crucial information about left ventricular function, ejection fraction, and the presence of any valvular abnormalities or wall motion abnormalities. It helps quantify the extent of myocardial damage and assess the severity of left ventricular dysfunction.
-
Hemodynamic Monitoring: Hemodynamic monitoring, including invasive arterial blood pressure and central venous pressure measurements, provides accurate assessment of cardiac output, systemic vascular resistance, and filling pressures. This helps quantify the severity of shock and guide treatment.
Management of Cardiogenic Shock Post-AMI
The management of cardiogenic shock following AMI is a multidisciplinary approach involving intensive care unit (ICU) management and requires immediate and aggressive intervention. The primary goals are to improve cardiac output, restore tissue perfusion, and support vital organ function. Key interventions include:
-
Oxygen Therapy: Supplemental oxygen is crucial to improve tissue oxygenation.
-
Intravenous Fluids: Careful fluid administration may be necessary to maintain adequate blood volume, but excessive fluid can exacerbate pulmonary edema.
-
Inotropic Support: Inotropic agents, such as dobutamine and milrinone, are used to improve myocardial contractility and increase cardiac output.
-
Vasopressor Support: Vasopressors, such as norepinephrine and dopamine, may be necessary to maintain blood pressure and improve tissue perfusion. These agents must be used judiciously to avoid excessive vasoconstriction, which can further compromise myocardial oxygen supply.
-
Mechanical Circulatory Support: In severe cases, mechanical circulatory support devices, such as intra-aortic balloon pump (IABP) or extracorporeal membrane oxygenation (ECMO), are used to temporarily assist the heart in pumping blood and improve organ perfusion. These devices provide temporary support, buying time for the heart to recover or for more definitive treatment.
-
Percutaneous Coronary Intervention (PCI): PCI, also known as angioplasty and stenting, is a minimally invasive procedure to open blocked coronary arteries and restore blood flow to the infarcted area. PCI is a cornerstone of acute myocardial infarction treatment and may be crucial in managing cardiogenic shock. Prompt reperfusion of the ischemic myocardium can limit infarct size and improve cardiac function.
-
Surgical Intervention: In selected cases, surgical intervention may be necessary to address complications such as ventricular septal rupture or papillary muscle rupture. Surgical repair can stabilize the cardiac anatomy and improve hemodynamic stability.
-
Symptom Management: Pain relief, anxiety reduction, and meticulous monitoring of fluid balance, electrolyte levels, and renal function are essential components of cardiogenic shock management.
Prognosis and Prevention
The prognosis for cardiogenic shock post-AMI remains poor despite advances in medical technology. Mortality rates remain high. Early recognition, aggressive management, and close monitoring are crucial for improving outcomes.
Preventing cardiogenic shock involves managing underlying risk factors for AMI, including:
-
Controlling hypertension: Effective management of high blood pressure reduces the risk of AMI.
-
Managing hyperlipidemia: Controlling high cholesterol levels prevents atherosclerosis and reduces the risk of coronary artery disease.
-
Smoking cessation: Quitting smoking significantly reduces the risk of coronary artery disease and AMI.
-
Diabetes management: Strict control of blood sugar levels is important for preventing vascular complications.
-
Regular exercise: Regular physical activity promotes cardiovascular health and reduces the risk of AMI.
-
Healthy diet: A balanced diet low in saturated and trans fats reduces the risk of atherosclerosis and coronary artery disease.
Conclusion
Cardiogenic shock following AMI represents a severe complication demanding immediate and aggressive management. Understanding the underlying causes and mechanisms, coupled with prompt diagnosis and appropriate treatment strategies, is essential for improving patient outcomes. While the prognosis remains challenging, advancements in medical technology and treatment protocols continue to improve survival rates and offer hope for affected patients. Prevention through lifestyle modifications and proactive management of risk factors remains a key strategy in mitigating the risk of this life-threatening complication.
Latest Posts
Latest Posts
-
Which Best Describes The Terrorist Planning Cycle
Mar 18, 2025
-
Cdl Combination Test Questions And Answers Pdf
Mar 18, 2025
-
Life Insurance Exam Questions And Answers Pdf
Mar 18, 2025
-
The Direct Carry Is Used To Transfer A Patient
Mar 18, 2025
-
The Emancipation Proclamation Of January 1 1863 Quizlet
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Cardiogenic Shock Following Ami Is Caused By Quizlet . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
