Explain Why Water Is Referred To As The Universal Solvent
Breaking News Today
Mar 31, 2025 · 5 min read
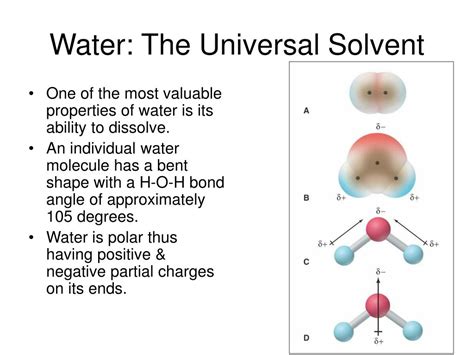
Table of Contents
Why Water Is Called the Universal Solvent: A Deep Dive into its Properties
Water, a seemingly simple molecule composed of two hydrogen atoms and one oxygen atom (H₂O), plays a crucial role in sustaining life on Earth. Its unique properties, however, extend far beyond its biological significance. One of the most remarkable characteristics of water is its ability to dissolve a vast array of substances, earning it the title of the universal solvent. But why is water so effective at dissolving so many different compounds? This article delves deep into the science behind water's solvent capabilities, exploring the intermolecular forces, polarity, and hydrogen bonding that make it such a remarkable substance.
The Polarity of Water: A Key to its Solvency
The secret to water's dissolving prowess lies in its polarity. Unlike nonpolar molecules, which have an even distribution of electrical charge, water molecules possess a distinct positive and negative end. This polarity arises from the difference in electronegativity between oxygen and hydrogen atoms. Oxygen, being more electronegative, attracts the shared electrons more strongly, creating a slight negative charge (δ-) on the oxygen atom and a slight positive charge (δ+) on the hydrogen atoms. This uneven charge distribution makes water a dipole – a molecule with two poles of opposite charge.
How Polarity Facilitates Dissolution
This polarity is crucial for dissolving many substances, particularly ionic compounds and polar molecules. Ionic compounds, like table salt (NaCl), are composed of positively charged cations (Na⁺) and negatively charged anions (Cl⁻) held together by strong electrostatic forces. When salt is added to water, the polar water molecules surround the ions, with the slightly negative oxygen atoms attracting the positive sodium ions and the slightly positive hydrogen atoms attracting the negative chloride ions. This process, called hydration, weakens the ionic bonds holding the salt crystal together and allows the ions to separate and dissolve in the water.
Polar molecules, similar to water, also possess regions of positive and negative charge. When a polar molecule is introduced to water, the positive and negative poles of both molecules attract each other, leading to dissolution. The strength of the interaction depends on the polarity of the solute molecule, with more polar molecules dissolving more readily in water.
Hydrogen Bonding: Strengthening Water's Solvent Power
Water's exceptional solvent abilities are further enhanced by its capacity to form hydrogen bonds. A hydrogen bond is a special type of dipole-dipole interaction that occurs between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. In water, the slightly positive hydrogen atoms of one molecule are attracted to the slightly negative oxygen atoms of another molecule, forming a network of hydrogen bonds.
The Network Effect
This extensive network of hydrogen bonds significantly influences water's properties. It contributes to its high boiling point, surface tension, and viscosity. Furthermore, it plays a crucial role in its ability to dissolve many substances. The hydrogen bonds help to stabilize the hydrated ions and polar molecules, keeping them dispersed throughout the solution and preventing them from re-associating.
The Limits of Water's "Universality"
While water is indeed a remarkably effective solvent, it's crucial to understand that its "universality" is not absolute. There are many substances that water cannot dissolve, or dissolves only to a very limited extent. These include:
Nonpolar Substances
Nonpolar substances, such as oils and fats, are composed of molecules with an even distribution of electrical charge. Since water molecules are polar, they have little attraction to nonpolar molecules. As a result, nonpolar substances tend to be insoluble or poorly soluble in water. This is often expressed by the saying "like dissolves like." Polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes.
Large, Complex Molecules
Even some polar molecules may not dissolve readily in water if they are very large or complex. The large size and intricate structure can hinder the interaction with water molecules, preventing complete dissolution. This is particularly true for many biological macromolecules, such as proteins and polysaccharides.
Substances with Strong Intermolecular Forces
Substances with strong intermolecular forces, such as ionic compounds with highly charged ions or molecules with strong hydrogen bonds, may have limited solubility in water. The strong attractive forces within these substances may be stronger than the attractive forces between the solute and water molecules.
Applications of Water's Solvency
Water's remarkable solvent properties have far-reaching applications in various fields:
Biological Systems
Water's ability to dissolve a wide range of substances is essential for life. It acts as a medium for transporting nutrients, removing waste products, and facilitating biochemical reactions within cells. The dissolving power of water allows for the transport of dissolved minerals and nutrients within plants, ensuring their survival and growth. In animals, this includes transporting oxygen through blood and helping to regulate body temperature.
Industrial Processes
Water is extensively used in industrial processes, such as cleaning, rinsing, and dissolving various chemicals. It plays a vital role in manufacturing, agriculture, and energy production. Its solvent properties are exploited in many industrial processes, from dissolving raw materials to extracting valuable products.
Environmental Science
Understanding water's solvent properties is critical in environmental science, particularly when studying water pollution. The ability of water to dissolve pollutants highlights the importance of maintaining water quality and preventing contamination. This knowledge helps in developing strategies for water purification and treatment. Understanding how contaminants dissolve and interact with water is crucial for environmental remediation efforts.
Everyday Life
The solvent properties of water are integral to our daily lives. We use water to clean ourselves, our clothes, and our homes. It's essential in food preparation, beverage production, and many other aspects of modern living. Its universal solvent properties make it the backbone of life and an essential part of everyday activities.
Conclusion: The Significance of Water's Solvency
Water's ability to dissolve a vast array of substances stems from its unique molecular structure, particularly its polarity and capacity for hydrogen bonding. While its "universality" has limitations, its exceptional solvent properties are fundamental to many natural processes and technological applications. Understanding the science behind water's solvency allows us to appreciate its significance in biological systems, industrial processes, environmental management, and our daily lives. The multifaceted nature of water's dissolving power continues to be a subject of fascination and scientific exploration, driving innovation and advancements across many fields. From the microscopic world of cellular processes to the vast scale of industrial applications, the universal solvent remains a cornerstone of our world.
Latest Posts
Latest Posts
-
Load Chart Values Can Pinpoint Failures Of
Apr 02, 2025
-
What Must You Do If You Suspect Iphone Efb Tampering
Apr 02, 2025
-
How Was Most Solid Waste Handled In The Middle Ages
Apr 02, 2025
-
Jonas Is A Whole Life Insurance Policyowner
Apr 02, 2025
-
The Number Of Subordinates That One Supervisor Can Manage Effectively
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Explain Why Water Is Referred To As The Universal Solvent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
