The Two Important Goals Of The Ich E6 Standard Are
Breaking News Today
Mar 15, 2025 · 7 min read
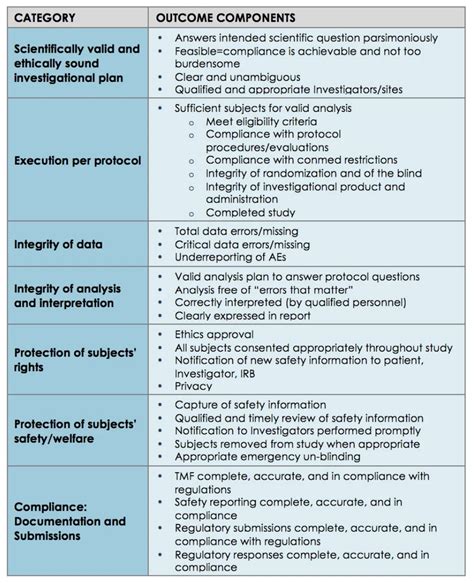
Table of Contents
- The Two Important Goals Of The Ich E6 Standard Are
- Table of Contents
- The Two Important Goals of the ICH E6 Standard: Achieving Good Clinical Practice (GCP) and Protecting Human Rights
- Ensuring the Rights, Safety, and Well-being of Trial Participants: A Foundation of Ethical Clinical Research
- 1. Informed Consent: The Cornerstone of Ethical Participation
- 2. Data Privacy and Confidentiality: Protecting Sensitive Information
- 3. Monitoring Safety: Proactive Risk Management
- 4. Investigator Responsibilities: Upholding Ethical Standards
- Ensuring the Credibility and Integrity of Clinical Trial Data: The Foundation of Sound Scientific Practice
- 1. Protocol Adherence: A Structured Approach to Data Collection
- 2. Data Management: Maintaining Data Accuracy and Completeness
- 3. Quality Control and Quality Assurance: Ensuring Data Reliability
- 4. Investigator Training and Qualification: Competence in Research Execution
- 5. Documentation: The Foundation of Transparency and Accountability
- The Interdependence of the Two Goals: A Holistic Approach to Clinical Research
- Conclusion: The Ongoing Evolution of ICH E6 and Its Enduring Importance
- Latest Posts
- Latest Posts
- Related Post
The Two Important Goals of the ICH E6 Standard: Achieving Good Clinical Practice (GCP) and Protecting Human Rights
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E6 guideline, titled "Good Clinical Practice (GCP)", is a cornerstone of ethical and scientific conduct in clinical trials. While encompassing numerous aspects of clinical research, its two overarching goals are paramount: ensuring the rights, safety, and well-being of trial participants and ensuring the credibility and integrity of clinical trial data. These goals are inextricably linked; without safeguarding participant welfare, the data generated is ethically compromised and scientifically questionable. Conversely, rigorous data collection processes contribute to the protection of participants by providing accurate information about the safety and efficacy of investigational products.
Ensuring the Rights, Safety, and Well-being of Trial Participants: A Foundation of Ethical Clinical Research
This goal forms the ethical bedrock of ICH E6. It dictates that every clinical trial must be conducted ethically, with the utmost respect for the rights and welfare of individuals involved. This includes:
1. Informed Consent: The Cornerstone of Ethical Participation
Informed consent is not simply a signature on a document; it's a process. ICH E6 emphasizes the importance of obtaining truly informed consent from each participant. This necessitates:
- Comprehensive Information: Participants must receive clear, concise, and understandable information about the trial, including its purpose, procedures, potential benefits and risks, their rights, and the voluntary nature of their participation. This information must be presented in a language they understand.
- Voluntary Participation: Participation must be entirely voluntary, without coercion or undue influence. Participants must be free to withdraw from the trial at any time without penalty.
- Capacity to Consent: Participants must possess the capacity to understand the information provided and make a reasoned decision about participation. This includes consideration of age, cognitive abilities, and any other factors that might impair comprehension.
2. Data Privacy and Confidentiality: Protecting Sensitive Information
Maintaining the confidentiality of participant data is crucial. ICH E6 mandates robust procedures to protect the privacy of personal information collected during the trial. This includes:
- Secure Data Handling: Data must be stored securely, using appropriate safeguards to prevent unauthorized access, use, or disclosure.
- Anonymization and De-identification: Whenever possible, data should be anonymized or de-identified to protect the identity of participants.
- Compliance with Data Privacy Regulations: All data handling procedures must comply with applicable data privacy regulations and laws, such as HIPAA in the United States or GDPR in Europe.
3. Monitoring Safety: Proactive Risk Management
Throughout the trial, participant safety must be continuously monitored. This includes:
- Adverse Event Reporting: Any adverse events (unexpected or undesirable effects) experienced by participants must be reported promptly and thoroughly.
- Safety Monitoring Committees: Independent safety monitoring committees may be established to review safety data regularly and provide recommendations to the sponsor.
- Early Termination of Participation: If a participant experiences a serious adverse event or shows signs of significant risk, their participation may be terminated early for their safety.
4. Investigator Responsibilities: Upholding Ethical Standards
The investigator plays a crucial role in ensuring participant safety and well-being. ICH E6 outlines the responsibilities of the investigator, including:
- Obtaining Informed Consent: The investigator is responsible for ensuring that informed consent is obtained properly.
- Monitoring Participant Safety: The investigator must monitor participants for any adverse events and take appropriate action.
- Maintaining Accurate Records: The investigator must maintain accurate and complete records of all trial activities.
- Adherence to Protocol: The investigator must adhere to the approved trial protocol and any amendments.
Ensuring the Credibility and Integrity of Clinical Trial Data: The Foundation of Sound Scientific Practice
This goal focuses on the reliability and trustworthiness of the data generated. The integrity of the data is essential for accurate interpretation of the results, the development of safe and effective therapies, and regulatory decision-making. This involves:
1. Protocol Adherence: A Structured Approach to Data Collection
The clinical trial protocol is the blueprint for the entire study. Strict adherence to the protocol ensures that the data collected is consistent, comparable, and reliable. Any deviations from the protocol must be documented and justified.
2. Data Management: Maintaining Data Accuracy and Completeness
Rigorous data management practices are crucial to maintain the accuracy and completeness of the data. This includes:
- Data Validation: Data should be validated to ensure its accuracy and consistency.
- Data Cleaning: Data should be cleaned to remove any errors or inconsistencies.
- Audit Trail: A comprehensive audit trail should be maintained to track all changes made to the data.
- Data Storage: Secure, reliable data storage mechanisms are essential to prevent data loss or corruption.
3. Quality Control and Quality Assurance: Ensuring Data Reliability
Quality control (QC) and quality assurance (QA) processes ensure the reliability of the data and the overall conduct of the trial. This involves:
- Regular Monitoring: Regular monitoring of the trial by qualified personnel is necessary to identify and address any potential issues.
- Audits and Inspections: Independent audits and inspections may be conducted to assess the quality of the trial and the reliability of the data.
- Corrective and Preventive Actions: Corrective and preventive actions should be implemented to address any identified deficiencies.
4. Investigator Training and Qualification: Competence in Research Execution
Investigators and their staff must be appropriately trained and qualified to conduct the trial in accordance with GCP principles. This ensures that data is collected accurately and reliably. Training should cover relevant aspects of:
- Protocol Understanding: A deep comprehension of the trial's objectives and methodology.
- Informed Consent Processes: Proper techniques for obtaining and documenting informed consent.
- Adverse Event Reporting: Accurate and timely reporting of adverse events.
- Data Management Practices: Correct procedures for data collection, recording, and storage.
- Regulatory Compliance: Familiarity with relevant regulations and guidelines.
5. Documentation: The Foundation of Transparency and Accountability
Meticulous documentation is essential for maintaining the integrity of the trial and supporting its findings. All aspects of the trial must be properly documented, including:
- Protocol and Amendments: The trial protocol and any amendments must be clearly documented.
- Informed Consent Forms: Informed consent forms must be properly documented and signed.
- Case Report Forms (CRFs): CRFs must be accurately and completely filled out.
- Adverse Event Reports: All adverse events must be promptly and accurately reported.
- Deviations and Discrepancies: Any deviations from the protocol must be clearly documented and justified.
The Interdependence of the Two Goals: A Holistic Approach to Clinical Research
The two goals of ICH E6 are not mutually exclusive; they are fundamentally intertwined. Protecting the rights and well-being of participants is crucial for generating reliable data. Participants who are not treated ethically are less likely to adhere to the protocol, leading to incomplete or inaccurate data. Conversely, rigorous data collection practices help protect participants by ensuring that safety data is accurately monitored and reported, allowing for timely interventions if needed.
For instance, a thorough informed consent process that ensures participants fully understand the study's risks and benefits enhances their trust in the research process. This, in turn, can lead to better adherence to the trial protocol, resulting in higher quality data. Similarly, effective safety monitoring can quickly identify and address potential issues that might jeopardize participant safety, strengthening both ethical conduct and data integrity.
Conclusion: The Ongoing Evolution of ICH E6 and Its Enduring Importance
ICH E6 is not a static document; it has been updated over time to reflect advancements in clinical research practices and technology. However, the core principles of protecting participants and ensuring data integrity remain central to its purpose. The guideline serves as a vital framework for ethical and scientific conduct, ensuring that clinical trials are conducted responsibly and contribute to the development of safe and effective therapies for patients worldwide. The continued adherence to and evolution of ICH E6 remains critical for maintaining public trust in clinical research and upholding the highest standards of scientific integrity. The ongoing refinement of GCP principles underscores the commitment to improving the ethical and scientific landscape of clinical research, ultimately benefiting both participants and the advancement of medical knowledge.
Latest Posts
Latest Posts
-
Which Is A Stroke Severity Tool That Helps Ems
Mar 16, 2025
-
Which Of The Following Is Not True About Unix
Mar 16, 2025
-
When Should A Dehydrating Product Be Applied To The Nail
Mar 16, 2025
-
Changes In Consumption And Gross Investment Can
Mar 16, 2025
-
Letrs Unit 3 Session 6 Check For Understanding
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about The Two Important Goals Of The Ich E6 Standard Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
