Thiols Have Structures Similar To Alcohols Except That They Contain
Breaking News Today
Mar 25, 2025 · 6 min read
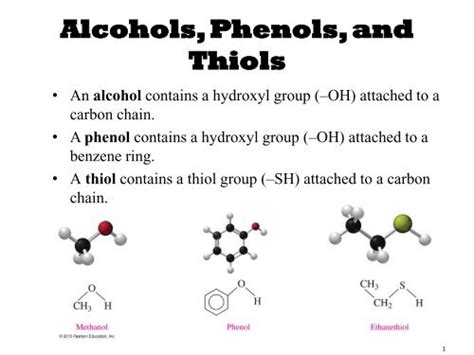
Table of Contents
Thiols: The Sulfur Analogs of Alcohols – Structure, Properties, and Applications
Thiols, also known as mercaptans, are organic compounds characterized by the presence of a sulfhydryl group (-SH). This functional group is analogous to the hydroxyl group (-OH) found in alcohols, but with sulfur replacing oxygen. This seemingly small substitution leads to significant differences in both the chemical and physical properties of thiols compared to their alcohol counterparts. Understanding these differences is crucial in various scientific fields, from biochemistry to materials science. This comprehensive article delves into the structure, properties, reactions, and diverse applications of thiols.
Structural Similarities and Differences between Thiols and Alcohols
The fundamental structural similarity between thiols and alcohols lies in the presence of a single atom bonded to a carbon atom. In alcohols, this atom is oxygen, forming a hydroxyl group (-OH); in thiols, it's sulfur, forming a sulfhydryl group (-SH). This seemingly minor difference, however, drastically alters the reactivity and properties of the molecule.
Structural Formulae and Nomenclature
Alcohols are named by replacing the "-e" ending of the corresponding alkane with "-ol." For example, CH₃OH is methanol. Thiols follow a similar naming convention, but with the suffix "-thiol" added to the parent alkane name. For example, CH₃SH is methanethiol. Alternatively, the prefix "mercapto-" can be used. Thus, CH₃SH can also be named methyl mercaptan.
Example:
- Alcohol: CH₃CH₂OH (Ethanol)
- Thiol: CH₃CH₂SH (Ethanethiol or Ethyl mercaptan)
Bond Length and Bond Strength
The C-S bond in thiols is longer than the C-O bond in alcohols. This is because sulfur is a larger atom than oxygen, leading to a greater atomic radius and a weaker bond. The S-H bond in thiols is also weaker than the O-H bond in alcohols, impacting their reactivity. This weaker S-H bond is responsible for many of the unique properties and reactions of thiols.
Electronegativity and Polarity
Oxygen is significantly more electronegative than sulfur. As a result, the O-H bond in alcohols is much more polar than the S-H bond in thiols. This difference in polarity greatly influences the intermolecular forces and solubility characteristics of thiols and alcohols. Alcohols exhibit stronger hydrogen bonding due to the higher electronegativity of oxygen. Thiols, while capable of weak hydrogen bonding, primarily exhibit weaker van der Waals forces.
Physical Properties of Thiols
The weaker intermolecular forces in thiols lead to several distinct physical properties when compared to alcohols:
Lower Boiling Points
Thiols generally have lower boiling points than alcohols with a similar molecular weight. This is a direct consequence of the weaker intermolecular forces present in thiols. The lack of strong hydrogen bonding means less energy is required to overcome the intermolecular attractions and transition to the gaseous phase.
Lower Solubility in Water
Due to the reduced polarity of the S-H bond, thiols exhibit lower solubility in water compared to alcohols. While small thiols might exhibit some solubility, larger thiols are generally insoluble in water. The inability to form strong hydrogen bonds with water molecules is the primary reason for this poor solubility.
Characteristic Odor
Thiols are notoriously known for their foul-smelling odor, often described as resembling rotten eggs or cabbage. This pungent odor is attributed to the sulfur atom and is often much more intense than the odor of the corresponding alcohol. The odor is so strong that thiols are added to natural gas to detect leaks, serving as a safety precaution.
Chemical Properties and Reactions of Thiols
The weaker S-H bond and lower electronegativity of sulfur compared to oxygen greatly influence the reactivity of thiols.
Oxidation Reactions
Thiols are easily oxidized, forming disulfides. This oxidation reaction involves the formation of a disulfide bond (-S-S-) between two thiol molecules. This is a crucial reaction in biochemistry, particularly in protein structure and function. The oxidation of thiols can be achieved using various oxidizing agents, including iodine, hydrogen peroxide, and air.
Example:
2 R-SH ⇌ R-S-S-R + 2H⁺ + 2e⁻
Reaction with Heavy Metals
Thiol groups have a high affinity for heavy metal ions. They can form stable complexes with metals such as mercury, lead, and cadmium. This property is exploited in various applications, including the detoxification of heavy metals and the development of metal-chelating agents.
Nucleophilic Reactions
Thiols are good nucleophiles, readily participating in nucleophilic substitution and addition reactions. The sulfur atom in the -SH group carries a partial negative charge and is capable of attacking electrophilic centers.
Reaction with Alkyl Halides
Thiols react with alkyl halides to form thioethers (sulfides) through a nucleophilic substitution reaction. This reaction is commonly used to synthesize thioethers with specific properties.
Applications of Thiols
The unique properties of thiols make them valuable in a variety of applications:
Biochemistry and Medicine
- Protein Structure: Disulfide bonds formed between cysteine residues (thiols) are crucial for maintaining the three-dimensional structure and stability of many proteins.
- Antioxidants: Some thiols act as antioxidants, protecting cells from damage caused by reactive oxygen species.
- Enzyme Cofactors: Thiols are important components of various enzymes, playing vital roles in their catalytic activity.
- Drug Development: Thiols are incorporated into many drugs, often modifying their properties or enhancing their bioavailability.
- Medical Imaging: Radiolabeled thiols are used in medical imaging techniques for visualizing certain organs or tissues.
Industrial Applications
- Natural Gas Odorant: Thiols, particularly tert-butylthiol, are added to natural gas to provide a distinctive and easily detectable odor, enhancing safety.
- Polymer Chemistry: Thiols are used in polymer chemistry to modify the properties of polymers or introduce specific functionalities.
- Wastewater Treatment: Thiols can be used in wastewater treatment to remove heavy metals.
- Chemical Synthesis: Thiols serve as important building blocks in the synthesis of a wide range of organic compounds.
Environmental Applications
- Heavy Metal Remediation: The ability of thiols to chelate heavy metals is being explored for environmental remediation purposes.
Safety Precautions
Many thiols are toxic and have a strong, unpleasant odor. Proper handling and safety measures are essential when working with thiols. Always wear appropriate personal protective equipment, including gloves, eye protection, and respiratory protection. Work in a well-ventilated area to minimize exposure to the pungent odor.
Conclusion
Thiols, despite their structural similarity to alcohols, possess unique chemical and physical properties driven by the differences between oxygen and sulfur. The weaker S-H bond, lower polarity, and characteristic odor distinguish them from alcohols. Their diverse applications span various fields, highlighting their importance in biochemistry, medicine, industry, and environmental science. Further research into thiols and their derivatives promises to unveil even more applications and functionalities in the future. Understanding their unique properties is essential for researchers and professionals working across numerous disciplines.
Latest Posts
Latest Posts
-
Traditional Savings Account Typical Add To Balance Regularly
Mar 28, 2025
-
Costs That Can Be Traced Directly To A Segment
Mar 28, 2025
-
Blank Refers To The Soil Removed From An Excavation
Mar 28, 2025
-
How Can Producers Maximize Their Profit Check All That Apply
Mar 28, 2025
-
In Each Succeeding Payment On An Installment Note The Amount
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Thiols Have Structures Similar To Alcohols Except That They Contain . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
