What Is The Iupac Name For The Compound Shown
Breaking News Today
Mar 24, 2025 · 7 min read
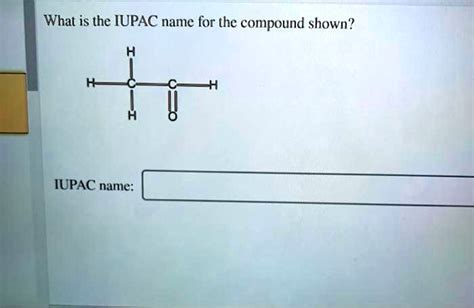
Table of Contents
Decoding Chemical Structures: A Deep Dive into IUPAC Nomenclature
Naming chemical compounds might seem like a daunting task, especially when confronted with complex structures. However, the International Union of Pure and Applied Chemistry (IUPAC) has established a systematic nomenclature system to ensure clarity and unambiguous communication within the scientific community. This article delves into the principles of IUPAC nomenclature, providing a comprehensive guide to naming organic and inorganic compounds, with a specific focus on understanding how to derive the IUPAC name for a given chemical structure. We'll explore the rules, strategies, and nuances involved, enabling you to confidently tackle even the most intricate molecular structures.
The Foundation of IUPAC Nomenclature: A Systematic Approach
IUPAC nomenclature is built upon a hierarchical system, prioritizing clarity and avoiding ambiguity. The process involves identifying the parent chain or structure, identifying functional groups and substituents, assigning locants (numbers indicating the position of substituents), and finally, combining these elements to construct the complete IUPAC name. This systematic approach ensures that every chemical compound receives a unique and easily understandable name.
Identifying the Parent Chain or Structure: The Backbone of the Molecule
The first crucial step in assigning an IUPAC name is identifying the parent chain or structure. This is usually the longest continuous carbon chain in the molecule, or the most stable and highly substituted ring system. For example, in a branched alkane, the longest continuous chain forms the parent alkane. In cyclic compounds, the largest ring system constitutes the parent structure. Special considerations apply to complex structures with multiple rings or functional groups, often requiring careful analysis to select the appropriate parent structure.
Identifying Functional Groups and Substituents: The Modifiers of the Parent Structure
Once the parent chain or structure is identified, the next step involves identifying any functional groups and substituents present. Functional groups are specific groups of atoms within a molecule that are responsible for its characteristic chemical reactions. Examples include alcohols (-OH), ketones (=O), carboxylic acids (-COOH), and amines (-NH2). Substituents are atoms or groups of atoms that replace hydrogen atoms on the parent chain or structure. These are typically alkyl groups (e.g., methyl, ethyl, propyl) or halogens (e.g., chloro, bromo, iodo). Proper identification of functional groups and substituents is vital in determining the correct IUPAC name.
Assigning Locants: Precisely Locating Substituents and Functional Groups
Locants are numbers used to specify the positions of substituents and functional groups on the parent chain or structure. Numbering should be done in a way that gives the lowest possible set of numbers to the substituents. For example, if a substituent is present on the second carbon atom, it's denoted as "2-methyl". In branched structures, the numbering is determined to minimize the numbers associated with the substituents. For cyclic compounds, the numbering starts at a substituent with higher priority.
Combining the Elements: Constructing the Complete IUPAC Name
The final step involves combining the identified parent structure, substituents, and locants to generate the complete IUPAC name. The substituents are listed alphabetically (ignoring prefixes like di-, tri-, etc. for alphabetization purposes, but including them in the final name), with their corresponding locants. The parent chain's name is appended at the end. For example, a compound with a six-carbon chain and two methyl groups on carbons 2 and 4 would be named 2,4-dimethylhexane.
Specific Examples and Case Studies: Applying IUPAC Nomenclature
Let’s consider some specific examples to illustrate the process of determining IUPAC names:
Example 1: A Simple Alkane
Consider the branched alkane with the structure: CH3-CH(CH3)-CH2-CH3.
- Parent chain: The longest continuous carbon chain contains four carbon atoms, making it butane.
- Substituents: A methyl group (CH3) is attached to the second carbon atom.
- Locants: The methyl group is located at carbon 2.
- IUPAC name: 2-methylbutane
Example 2: A Compound with Multiple Substituents
Consider the compound with the structure: CH3-CH(CH3)-CH(CH2CH3)-CH3
- Parent chain: The longest continuous chain has four carbon atoms (butane).
- Substituents: A methyl group (CH3) on carbon 2 and an ethyl group (CH2CH3) on carbon 3.
- Locants: Methyl group at carbon 2, ethyl group at carbon 3. Alphabetization of substituents means "ethyl" comes before "methyl".
- IUPAC name: 3-ethyl-2-methylbutane
Example 3: A Cyclic Compound
Consider cyclohexane with a methyl group and a chlorine atom:
- Parent structure: Cyclohexane.
- Substituents: Methyl and chloro.
- Locants: Numbering starts arbitrarily, aiming for the lowest numbers. Let’s assign the methyl group to position 1.
- IUPAC name: 1-chloro-1-methylcyclohexane
Example 4: A Compound with a Functional Group (Alcohol)
Consider a compound with an alcohol functional group: CH3-CH(OH)-CH2-CH3
- Parent chain: Butane (4 carbon atoms).
- Functional group: Hydroxyl group (-OH) which indicates an alcohol.
- Locants: Hydroxyl group at position 2.
- IUPAC name: 2-butanol
Example 5: A Compound with a Ketone Functional Group
Consider a compound with a ketone functional group: CH3-CO-CH2-CH3
- Parent chain: Butane (4 carbon atoms).
- Functional Group: Ketone (=O), making it a butanone. The carbonyl group must be on the main chain, otherwise, it's a substituted aldehyde.
- Locants: The carbonyl group is on carbon 2. Since butanone has a carbonyl group on the second carbon as the smallest number, the 2 is not needed in the name.
- IUPAC name: Butanone
These examples illustrate the systematic application of IUPAC nomenclature rules to different types of organic compounds. The process might seem complex initially, but with practice and a systematic approach, it becomes straightforward and second nature.
Advanced Topics in IUPAC Nomenclature: Navigating Complex Structures
While the basic principles discussed above cover a significant portion of organic compounds, more complex structures necessitate a deeper understanding of IUPAC nomenclature rules. This section briefly touches upon some of these advanced concepts:
Stereochemistry: Describing the Three-Dimensional Arrangement
IUPAC nomenclature incorporates stereochemical descriptors (e.g., R/S, E/Z) to specify the three-dimensional arrangement of atoms in a molecule. These descriptors are crucial when dealing with chiral molecules (molecules that are not superimposable on their mirror images). Understanding stereochemistry and its representation in IUPAC names requires a good grasp of concepts such as chirality, enantiomers, and diastereomers.
Polyfunctional Compounds: Handling Multiple Functional Groups
When a compound contains multiple functional groups, a hierarchy of priority determines the parent functional group that determines the suffix of the IUPAC name. The higher priority functional group determines the main functional group, and other functional groups are treated as substituents. Specific rules govern the order of priority for various functional groups.
Heterocyclic Compounds: Naming Compounds with Heteroatoms
Heterocyclic compounds contain atoms other than carbon in their ring structures (e.g., nitrogen, oxygen, sulfur). Their nomenclature involves specific prefixes and suffixes to indicate the nature and position of the heteroatoms within the ring system.
Inorganic Compounds: A Different Set of Rules
The IUPAC nomenclature for inorganic compounds differs somewhat from that of organic compounds. It involves specifying the oxidation states of elements, using prefixes to indicate the number of atoms, and employing specific naming conventions for different classes of inorganic compounds (e.g., salts, acids, oxides).
Conclusion: Mastering IUPAC Nomenclature for Clear Scientific Communication
IUPAC nomenclature is essential for clear and unambiguous communication in chemistry. It provides a systematic and logical framework for naming chemical compounds, avoiding the confusion and ambiguity that would arise from using common names. By understanding the fundamental principles and applying the rules systematically, one can confidently name even complex chemical structures. Although the system might initially appear complex, with consistent practice and attention to detail, mastering IUPAC nomenclature becomes achievable, ensuring accuracy and effectiveness in all chemical endeavors. The importance of precise chemical communication cannot be overstated, and IUPAC nomenclature serves as the cornerstone of this crucial aspect of scientific work. Continuous learning and practice remain key to developing proficiency in this vital area.
Latest Posts
Latest Posts
-
Correctly Label The Features Of The Larynx
Mar 26, 2025
-
You May Have Found Your Purpose If
Mar 26, 2025
-
Insert A Built In Bibliography Without A Preformatted Heading
Mar 26, 2025
-
A Three Phase Induction Motor May Have
Mar 26, 2025
-
The Crucible Act 3 Questions And Answers Pdf
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Iupac Name For The Compound Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
