Which Of These Correctly Defines The Poh Of A Solution
Breaking News Today
Mar 17, 2025 · 5 min read
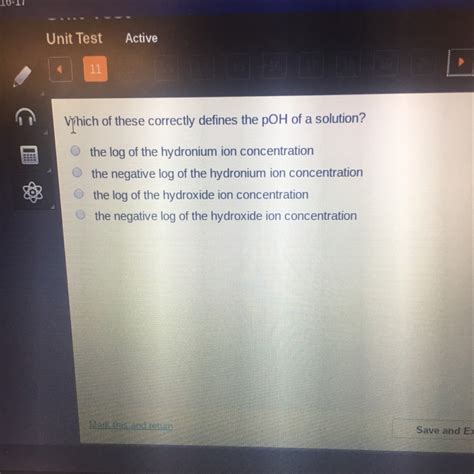
Table of Contents
Which of These Correctly Defines the pH of a Solution? Understanding Acidity, Alkalinity, and the pH Scale
The pH of a solution is a fundamental concept in chemistry with far-reaching implications across various fields, from environmental science and medicine to industrial processes and food technology. Understanding pH is crucial for interpreting chemical reactions, predicting behavior, and ensuring safety. But what exactly is pH, and how is it correctly defined? This comprehensive guide will delve into the intricacies of pH, explore common misconceptions, and provide a clear and concise definition.
What is pH?
At its core, pH is a measure of the hydrogen ion concentration ([H⁺]) in a solution. It's a logarithmic scale, meaning each whole number change represents a tenfold difference in acidity or alkalinity. This scale typically ranges from 0 to 14, although solutions can exist outside this range.
A lower pH indicates a higher concentration of H⁺ ions, signifying a more acidic solution. Conversely, a higher pH indicates a lower concentration of H⁺ ions, signifying a more alkaline (or basic) solution. A pH of 7 is considered neutral, representing a balance between H⁺ and hydroxide (OH⁻) ions.
The Correct Definition: Focusing on Hydrogen Ion Concentration
Many definitions attempt to explain pH, but the most accurate and concise definition centers on the hydrogen ion concentration:
pH = -log₁₀[H⁺]
This equation is the cornerstone of pH measurement. Let's break it down:
-
-log₁₀: This represents the negative base-10 logarithm. The logarithm function converts a wide range of hydrogen ion concentrations into a manageable scale of 0 to 14. The negative sign ensures that the pH scale increases as acidity decreases.
-
[H⁺]: This denotes the molar concentration of hydrogen ions in the solution, typically expressed in moles per liter (mol/L).
This mathematical definition accurately captures the essence of pH: a quantitative measure directly reflecting the concentration of hydrogen ions. Any definition that omits this core relationship is incomplete or potentially misleading.
Debunking Common Misconceptions
Several misconceptions surrounding pH definitions are prevalent:
-
pH as a measure of only H⁺ ions: While primarily focused on H⁺, pH implicitly reflects the balance between H⁺ and OH⁻ ions. This is because in aqueous solutions, the product of their concentrations ([H⁺][OH⁻]) is a constant (Kw) at a given temperature. Therefore, changes in [H⁺] directly impact [OH⁻], and vice versa.
-
pH as a measure of the strength of an acid or base: pH measures the concentration of H⁺ ions, not the strength (or dissociation constant) of the acid or base itself. A strong acid might have a lower pH than a weak acid at the same concentration because the strong acid dissociates more completely, releasing more H⁺ ions.
-
pH as a direct measure of acidity or alkalinity: pH is a quantitative measure that reflects acidity or alkalinity, but it doesn't directly define them qualitatively. The terms "acidic," "neutral," and "alkaline" are descriptors based on the pH value in relation to 7.
-
pH as a universal indicator of chemical reactivity: While pH is crucial for many reactions, it's not the only factor determining reactivity. Other parameters such as temperature, concentration of other reactants, and the presence of catalysts also play significant roles.
Understanding the pH Scale in Detail
The pH scale's logarithmic nature necessitates a detailed understanding of its implications:
-
Each whole number change represents a tenfold difference: A solution with a pH of 3 is ten times more acidic than a solution with a pH of 4, and one hundred times more acidic than a solution with a pH of 5.
-
The scale is not linear: The difference between pH 1 and pH 2 is much greater than the difference between pH 6 and pH 7.
-
The scale extends beyond 0 and 14: Extremely concentrated strong acids can exhibit negative pH values, and highly concentrated strong bases can exceed a pH of 14.
-
pH depends on temperature: The value of Kw, and thus the relationship between [H⁺] and [OH⁻], varies with temperature. Therefore, the pH of a solution can change slightly with temperature fluctuations.
Measuring pH: Practical Applications
The accurate measurement of pH is essential in numerous applications:
-
Water quality monitoring: pH is a critical indicator of water quality, affecting aquatic life and ecosystem health.
-
Soil analysis: Optimal plant growth depends on the soil's pH, influencing nutrient availability.
-
Industrial processes: Many industrial processes, including chemical manufacturing and food processing, require precise pH control for efficient and safe operation.
-
Medical diagnostics: Body fluid pH is crucial for maintaining physiological functions. Abnormalities in blood pH can indicate serious medical conditions.
-
Food and beverage industry: pH affects food preservation, taste, and texture. Accurate pH control is crucial for maintaining product quality and safety.
Examples of pH Values in Everyday Life
To illustrate the breadth of the pH scale and its relevance, let's examine some common substances:
-
Stomach acid: Highly acidic (pH ~1-3) due to hydrochloric acid.
-
Lemon juice: Acidic (pH ~2-3).
-
Vinegar: Acidic (pH ~2.5-3.5).
-
Rainwater: Slightly acidic (pH ~5.6) due to dissolved carbon dioxide.
-
Pure water: Neutral (pH ~7).
-
Baking soda solution: Slightly alkaline (pH ~8-9).
-
Household ammonia: Alkaline (pH ~11-12).
-
Sodium hydroxide solution: Highly alkaline (pH >12).
Conclusion: The Importance of Precise Definition
The precise definition of pH as -log₁₀[H⁺] is paramount for a clear understanding of this crucial chemical concept. Understanding the logarithmic nature of the pH scale and its implications for interpreting acidity, alkalinity, and chemical reactions is vital across a multitude of scientific disciplines and practical applications. Avoiding common misconceptions and appreciating the quantitative nature of pH are essential for accurate measurements and interpretations. By grasping the fundamental definition and its nuances, one can navigate the world of pH with greater confidence and competence.
Latest Posts
Latest Posts
-
True Or False Professional And Technical Communication Is Research Oriented
Mar 18, 2025
-
Which Best Describes The Terrorist Planning Cycle
Mar 18, 2025
-
Cdl Combination Test Questions And Answers Pdf
Mar 18, 2025
-
Life Insurance Exam Questions And Answers Pdf
Mar 18, 2025
-
The Direct Carry Is Used To Transfer A Patient
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Of These Correctly Defines The Poh Of A Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
