Atoms Share Electrons Unequally S An Blank Bond
Breaking News Today
Mar 16, 2025 · 6 min read
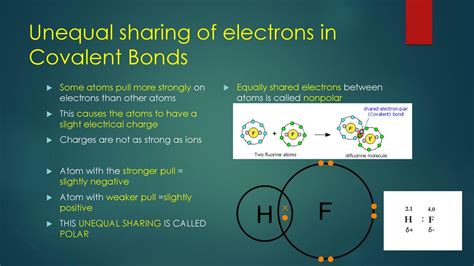
Table of Contents
Atoms Share Electrons Unequally: An Introduction to Polar Covalent Bonds
Atoms are the fundamental building blocks of matter, and their interactions determine the properties of all substances. One crucial interaction is the sharing of electrons between atoms to form chemical bonds. While the concept of electron sharing is central to covalent bonding, the equality of this sharing is not always the case. This article delves into the fascinating world of polar covalent bonds, where electrons are shared unequally between atoms, leading to a distribution of charge within the molecule. We'll explore the factors influencing polarity, the consequences of unequal electron sharing, and the importance of polar covalent bonds in various chemical and biological systems.
Understanding Covalent Bonds: A Foundation
Before diving into the intricacies of polar covalent bonds, let's briefly revisit the basics of covalent bonding. Covalent bonds form when two atoms share one or more pairs of electrons to achieve a more stable electron configuration, often resembling that of a noble gas. This sharing allows both atoms to complete their outermost electron shell (valence shell), reducing their overall energy and increasing stability.
Examples of non-polar covalent bonds include those found in diatomic molecules like H₂ (hydrogen gas) and O₂ (oxygen gas). In these cases, the atoms involved have very similar electronegativities, meaning they have a nearly equal attraction for the shared electrons. The electron density is fairly evenly distributed between the two atoms.
However, the world of chemical bonding is not always so symmetrical. Many molecules exhibit a more complex type of covalent bond, where the electron sharing is decidedly uneven.
Introducing Polar Covalent Bonds: Unequal Sharing
A polar covalent bond arises when two atoms with significantly different electronegativities share electrons. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Atoms with higher electronegativity exert a stronger pull on the shared electrons, resulting in an unequal distribution of electron density.
This unequal sharing creates a dipole moment, where one end of the bond carries a partial negative charge (δ-) and the other end carries a partial positive charge (δ+). The atom with the higher electronegativity attracts more electron density and acquires the partial negative charge, while the atom with the lower electronegativity experiences a deficiency of electron density and acquires the partial positive charge.
Think of it like a tug-of-war: If one player is significantly stronger (higher electronegativity), they'll pull the rope (electrons) closer to their side, creating an imbalance.
Factors Influencing Polarity
Several factors contribute to the polarity of a covalent bond:
1. Electronegativity Difference
The most crucial factor influencing bond polarity is the difference in electronegativity between the two atoms involved. The larger the difference, the more polar the bond becomes. A significant electronegativity difference leads to a substantial charge separation, resulting in a strongly polar bond. Conversely, a small electronegativity difference results in a less polar, or even non-polar, bond. The Pauling electronegativity scale is commonly used to quantify electronegativity values.
2. Atomic Size
The size of the atoms involved can also play a role. Larger atoms tend to have lower electronegativities because their valence electrons are further from the nucleus and experience less attraction. This can contribute to a larger electronegativity difference and increased bond polarity when paired with smaller, more electronegative atoms.
3. Bond Length
Bond length also influences polarity indirectly. Shorter bonds often lead to stronger interactions between the atoms and a greater influence of the more electronegative atom on the shared electrons, resulting in higher polarity.
Consequences of Unequal Electron Sharing
The unequal sharing of electrons in polar covalent bonds has several significant consequences:
1. Dipole Moment and Molecular Polarity
As mentioned earlier, the unequal electron distribution creates a dipole moment, indicated by a vector pointing from the positive to the negative end of the bond. The overall polarity of a molecule depends on the arrangement of polar bonds within the molecule and the cancellation or summation of individual dipole moments. Some molecules with polar bonds can have a zero net dipole moment if the bond dipoles cancel each other out due to symmetry (e.g., carbon dioxide, CO₂). Other molecules with polar bonds will have a net dipole moment, making the molecule polar overall (e.g., water, H₂O).
2. Intermolecular Forces
Polar molecules interact through stronger intermolecular forces than non-polar molecules. These forces, such as dipole-dipole interactions and hydrogen bonding (a special type of dipole-dipole interaction involving hydrogen), influence the physical properties of substances, such as melting point, boiling point, and solubility. Polar molecules tend to have higher melting and boiling points because more energy is required to overcome the stronger intermolecular attractions.
3. Solubility
Polar molecules tend to be soluble in polar solvents (like water), while non-polar molecules are soluble in non-polar solvents. This is based on the principle of "like dissolves like," where similar intermolecular forces facilitate dissolution.
Examples of Polar Covalent Bonds
Many biologically and chemically significant molecules contain polar covalent bonds:
-
Water (H₂O): The oxygen atom is significantly more electronegative than the hydrogen atoms, resulting in a highly polar molecule with a strong dipole moment. This polarity is essential for water's unique properties, making it an excellent solvent and crucial for life.
-
Carbon Monoxide (CO): Oxygen's higher electronegativity creates a polar bond, making CO a slightly polar molecule.
-
Hydrogen Fluoride (HF): Fluorine is the most electronegative element, leading to a highly polar bond in HF.
-
Ammonia (NH₃): Nitrogen's higher electronegativity compared to hydrogen creates polar N-H bonds, resulting in a polar molecule.
Applications and Importance
Polar covalent bonds are fundamental to the structure and function of countless molecules:
-
Biological Molecules: Proteins, carbohydrates, and nucleic acids, the building blocks of life, rely heavily on polar covalent bonds. These bonds contribute to the specific three-dimensional structures of these biomolecules, enabling their diverse biological functions. The polarity of these molecules also influences their interactions with water and other biomolecules.
-
Chemical Reactions: The polarity of molecules significantly influences their reactivity. Polar molecules readily participate in many chemical reactions, including acid-base reactions, nucleophilic substitutions, and addition reactions.
-
Materials Science: The properties of many materials are directly related to the presence and nature of polar covalent bonds. The polarity of the bonds determines the material's strength, electrical conductivity, and other physical properties.
Conclusion: The Significance of Unequal Sharing
The seemingly small difference in electron sharing in polar covalent bonds has profound consequences for the properties and behavior of molecules. Understanding the concept of electronegativity, its impact on bond polarity, and the resulting consequences for molecular interactions is crucial for comprehending the vast array of chemical and biological phenomena. From the unique properties of water to the complex structures of biological macromolecules, polar covalent bonds play a central and indispensable role in the world around us. Further exploration into the specifics of electronegativity trends within the periodic table and their application to predicting bond polarities can enhance our understanding and ability to predict molecular behavior in various contexts.
Latest Posts
Latest Posts
-
Which Action Requires An Organization To Carry Out A Pia
Mar 16, 2025
-
The Revenue Recognition Principle States That Revenue
Mar 16, 2025
-
The Cognitive Behavioral Approach To Therapy Stresses
Mar 16, 2025
-
Letrs Unit 5 Session 5 Check For Understanding
Mar 16, 2025
-
This Figure Depicts The Basic Anatomy Of The
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Atoms Share Electrons Unequally S An Blank Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
