What Is The Primary Buffer In The Plasma
Breaking News Today
Mar 27, 2025 · 5 min read
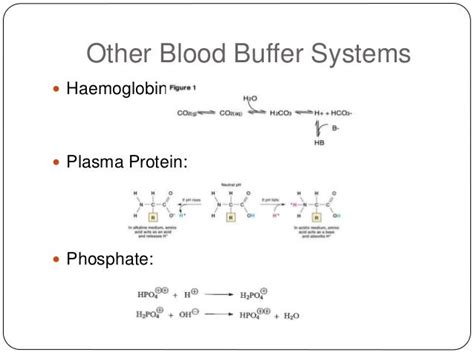
Table of Contents
What is the Primary Buffer in the Plasma? The Bicarbonate Buffer System Explained
The human body is a marvel of intricate biochemical processes, constantly striving to maintain a stable internal environment, a state known as homeostasis. One crucial aspect of homeostasis is maintaining a stable pH, typically within a narrow range of 7.35 to 7.45. Fluctuations outside this range can be life-threatening, leading to acidosis (low pH) or alkalosis (high pH). This tight pH regulation is largely achieved through the sophisticated interplay of various buffer systems, with the bicarbonate buffer system playing a primary role in plasma.
Understanding Buffers: The Body's pH Guardians
Before diving into the specifics of the bicarbonate buffer system, it's essential to grasp the fundamental concept of a buffer. A buffer is a solution that resists changes in pH upon the addition of small amounts of acid or base. This resistance is achieved through the presence of a weak acid and its conjugate base (or a weak base and its conjugate acid). When an acid is added to a buffered solution, the conjugate base reacts with it, minimizing the change in pH. Conversely, when a base is added, the weak acid reacts, again preventing significant pH alteration. This buffering capacity is crucial for maintaining the delicate pH balance in biological systems.
The Bicarbonate Buffer System: The Plasma's Primary Defense
The primary buffer in plasma is the bicarbonate buffer system, comprised of carbonic acid (H₂CO₃) and its conjugate base, bicarbonate (HCO₃⁻). This system's effectiveness stems from several factors:
1. Abundance of Components:
Both carbonic acid and bicarbonate are present in relatively high concentrations in plasma, providing a substantial buffering capacity. The bicarbonate concentration is typically much higher than the carbonic acid concentration, which is crucial for effectively neutralizing added acids.
2. Open System:
Unlike many other buffer systems, the bicarbonate buffer system is an "open" system, meaning it's constantly replenished and regulated. Carbon dioxide (CO₂) is constantly produced as a byproduct of cellular respiration. This CO₂ enters the bloodstream and reacts with water (H₂O) to form carbonic acid (H₂CO₃), which then dissociates into bicarbonate (HCO₃⁻) and hydrogen ions (H⁺). The lungs and kidneys play crucial roles in regulating the levels of CO₂ and bicarbonate, respectively, ensuring the system remains effective.
3. Renal and Respiratory Regulation:
The kidneys and lungs work in concert to maintain the delicate balance of the bicarbonate buffer system. The lungs regulate the partial pressure of carbon dioxide (PCO₂), influencing the concentration of carbonic acid. Increased ventilation (faster breathing) expels more CO₂, reducing H₂CO₃ and raising the pH. Decreased ventilation has the opposite effect. The kidneys regulate the excretion of bicarbonate ions. When the pH is too low (acidosis), the kidneys increase bicarbonate reabsorption and excrete more H⁺ ions. When the pH is too high (alkalosis), the kidneys increase bicarbonate excretion and reabsorb more H⁺ ions.
How the Bicarbonate Buffer System Works: A Step-by-Step Explanation
Let's explore how the bicarbonate buffer system neutralizes both acids and bases:
Neutralizing Acids:
When an acid (such as lactic acid produced during strenuous exercise) is added to the plasma, the bicarbonate ions (HCO₃⁻) react with the excess H⁺ ions to form carbonic acid (H₂CO₃):
H⁺ + HCO₃⁻ ↔ H₂CO₃
This reaction prevents a significant drop in pH. The carbonic acid then further dissociates into water and carbon dioxide:
H₂CO₃ ↔ H₂O + CO₂
The CO₂ is then exhaled by the lungs, further reducing the acidity.
Neutralizing Bases:
When a base is added to the plasma, the carbonic acid (H₂CO₃) reacts with the excess OH⁻ ions to form bicarbonate (HCO₃⁻) and water:
OH⁻ + H₂CO₃ ↔ HCO₃⁻ + H₂O
This reaction prevents a significant rise in pH. The increase in bicarbonate can be further regulated by the kidneys.
Other Plasma Buffers: Supporting Roles
While the bicarbonate buffer system is the primary buffer in plasma, other buffer systems contribute to maintaining pH homeostasis:
-
Phosphate Buffer System: This system involves dihydrogen phosphate (H₂PO₄⁻) and monohydrogen phosphate (HPO₄²⁻). It plays a smaller role in plasma but is more significant in intracellular fluids and urine.
-
Protein Buffer System: Plasma proteins, such as albumin, contain both acidic and basic groups. These groups can bind to either H⁺ or OH⁻ ions, buffering against pH changes. This system is particularly effective within cells and contributes to plasma buffering.
-
Hemoglobin Buffer System: Hemoglobin, the oxygen-carrying protein in red blood cells, is a significant buffer within erythrocytes and contributes indirectly to plasma buffering by affecting the CO₂ carrying capacity of blood.
Clinical Significance: When the Buffer System Fails
Disruptions to the bicarbonate buffer system can have severe clinical consequences, leading to acid-base imbalances. These imbalances can be caused by various factors, including:
-
Respiratory Acidosis: This occurs when the lungs cannot effectively remove CO₂, leading to an increase in H₂CO₃ and a decrease in pH. Causes include pneumonia, emphysema, and drug overdose.
-
Respiratory Alkalosis: This occurs when the lungs remove too much CO₂, leading to a decrease in H₂CO₃ and an increase in pH. Causes include hyperventilation (often due to anxiety or altitude sickness).
-
Metabolic Acidosis: This occurs due to an increase in non-carbonic acids in the body or a decrease in bicarbonate. Causes include diabetic ketoacidosis, lactic acidosis, and renal failure.
-
Metabolic Alkalosis: This occurs due to a loss of acid or gain of base in the body. Causes include vomiting, diuretic use, and ingestion of certain medications.
The accurate diagnosis and treatment of these conditions require careful assessment of blood gas levels, including pH, PCO₂, and bicarbonate concentration. Treatment may involve addressing the underlying cause, administering fluids, or administering medications to correct the acid-base imbalance.
Conclusion: The Importance of pH Homeostasis
Maintaining a stable plasma pH is essential for numerous physiological processes. The bicarbonate buffer system, working in conjunction with the respiratory and renal systems, effectively safeguards against potentially harmful pH fluctuations. Understanding the complexities of this system is crucial for comprehending the intricate mechanisms of homeostasis and for diagnosing and managing acid-base imbalances in clinical settings. The body’s remarkable ability to regulate pH underscores the importance of this essential physiological process in maintaining overall health and well-being. Further research into the intricacies of this system and the interaction between different buffer systems will continue to refine our understanding of this vital aspect of human physiology.
Latest Posts
Latest Posts
-
Match The Type Of Bond With Its Description
Mar 30, 2025
-
Level Premium Permanent Insurance Accumulates A Reserve That Will Eventually
Mar 30, 2025
-
An Employee Requested That The Balance Of Her 401k
Mar 30, 2025
-
Bluebottles And A Case Of Accidental Death
Mar 30, 2025
-
Thermal Radiation Gets Its Name Because
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Primary Buffer In The Plasma . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
